Preparation method of palbociclib crystal form A
A technology of palbocoxib and its crystal form, which is applied in the field of chemical synthesis, can solve problems such as difficulty in ensuring product purity, difficulty in compound purification, skin irritation, etc., and achieves simple and easy preparation methods, easy post-processing, and harm to the human body and the environment. less harmful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
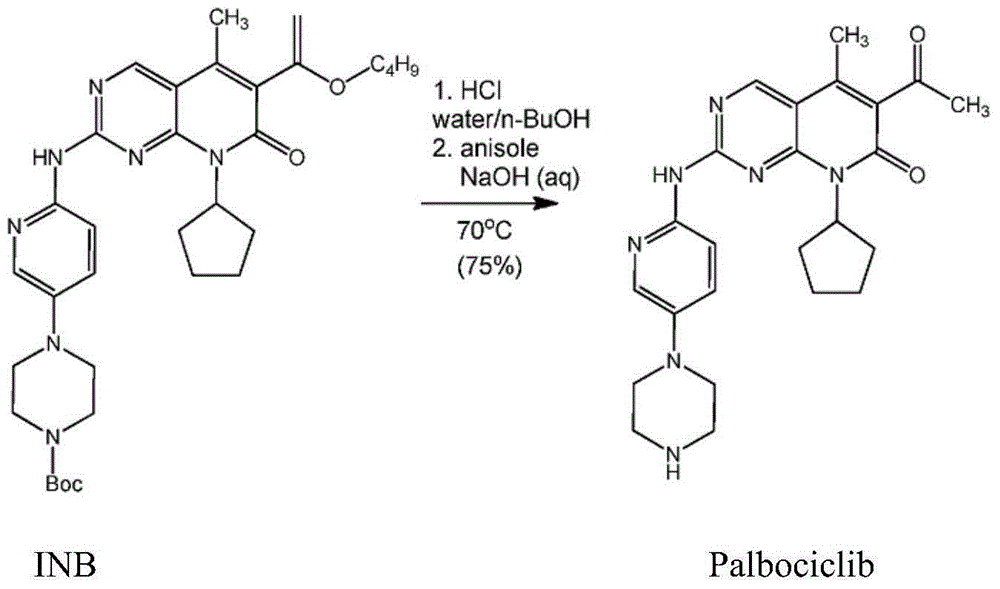
[0055] Example 1: Preparation of Salts of Palbociclib
[0056] Weigh 10 g of compound INB, add 230 ml of methanol and 3 ml of water. When the temperature was raised to 55°C, the solution was yellow and suspended; 8.5 grams of isethionic acid was added dropwise, and the solution gradually became clear; the temperature was raised to 65°C, and stirred for 12 hours; a large amount of solids were precipitated in the system, and filtered to obtain 10.02 grams of the product INC sample, the yield 86.4%.
Embodiment 2
[0057] Example 2: Preparation of Palbociclib Free Base Crystal Form A
[0058] Weigh 10 grams of the product INC of Example 1, add 150 milliliters of water and 30 milliliters of methanol, and the solution is clarified. After filtering, add sodium hydroxide solution (5% W / W) dropwise to the mother liquor to adjust the pH of the solution to >8. Stirring was continued at room temperature for 3 hours, a large amount of solid precipitated and was filtered. To obtain a solid, add 150 ml of water, stir for 1 hour, filter, and wash with water. After drying, 5.4 g of the product was obtained with a yield of 84.5%. 99.9% pure.
Embodiment 3
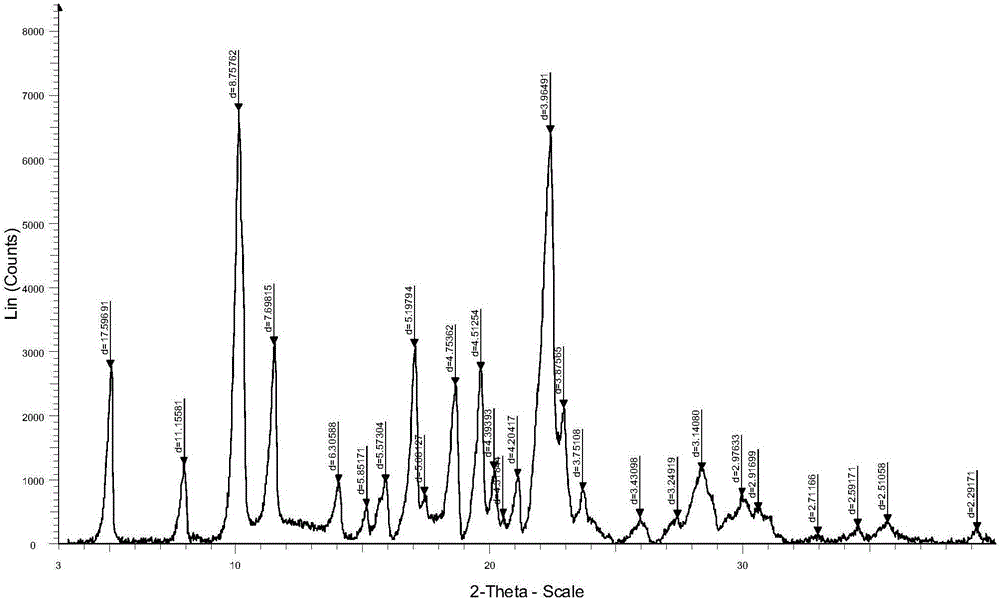
[0059] Embodiment 3: determination of crystal form
[0060] Detect Example 2 product powder X-ray diffraction (PXRD):
[0061] Use the Rigaku Dmax / 2400 type X-ray polycrystalline powder diffractometer (condition: Cu Kα, 40kV) to measure;
[0062] Please refer to the attached manual for the PXRD spectrum figure 1 :
[0063] Peak table of PXRD pattern:
[0064]
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com