Composition used for improving sialyl level of recombinant human type II tumor necrosis factor receptor-antibody fusion protein
A composition and cofactor technology, applied in the biological field, can solve problems such as human beings that have not yet been regulated, and achieve the effects of being suitable for large-scale production, increasing the speed of enzyme reactions, and increasing the supply of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
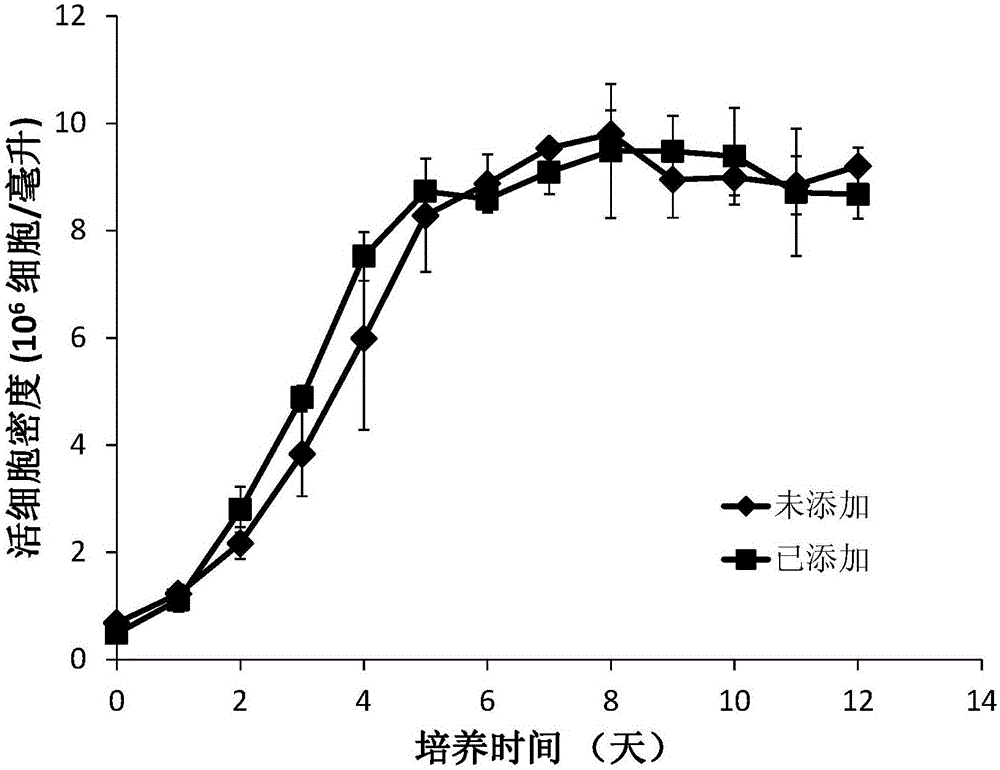
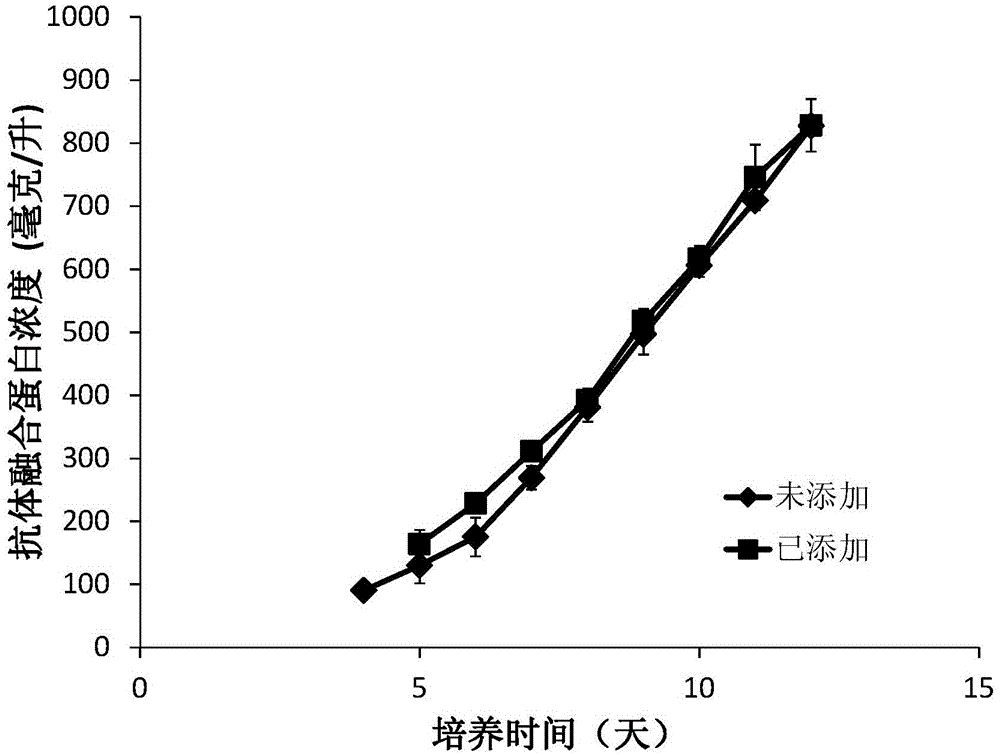
[0057] Example 1 is applied to the commercial serum-free medium EX-CELL produced by SAFC Biosciences in the United States in the batch culture process TM Composition of 302CHO for increasing the level of sialylation of recombinant human tumor necrosis factor receptor type II-antibody fusion protein.
[0058] Eighteen compositions for improving the sialylation level of recombinant human tumor necrosis factor receptor type II-antibody fusion protein are provided in this example (Table 2), containing manganese chloride tetrahydrate, galactose, glucosamine, N- Acetylmannose, N-acetylglucosamine, uracil, dexamethasone, and hydrocortisone.
[0059] The preparation method of the composition in this embodiment is as follows: fully dissolving the recipe amounts of manganese chloride tetrahydrate, galactose, glucosamine, N-acetylmannose, N-acetylglucosamine and uracil in pyrogen-free ultrapure water , Dissolve the prescribed amount of dexamethasone and hydrocortisone in 99% ethanol so...
Embodiment 2
[0069] Example 2 In the process of fed-batch culture, the composition for increasing the sialylation level of recombinant human tumor necrosis factor receptor type II-antibody fusion protein was applied to the CD optimal commercial basal medium developed by the American Life techonology company.
[0070] The composition used in this example for improving the sialylation level of recombinant human tumor necrosis factor receptor type II-antibody fusion protein contains:
[0071] Galactose: 30mmol / L
[0072] Glucosamine: 20mmol / L
[0073] N-acetylmannose: 20mmol / L
[0074] N-acetylglucosamine: 20mmol / L
[0075] Uracil: 20mmol / L
[0076] Manganese chloride tetrahydrate: 0.1mmol / L
[0077] Dexamethasone: 0.1mmol / L and
[0078] Hydrocortisone: 0.1 mmol / L.
[0079] The preparation method of the composition in this embodiment is as follows: fully dissolving the recipe amounts of manganese chloride tetrahydrate, galactose, glucosamine, N-acetylmannose, N-acetylglucosamine and ura...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com