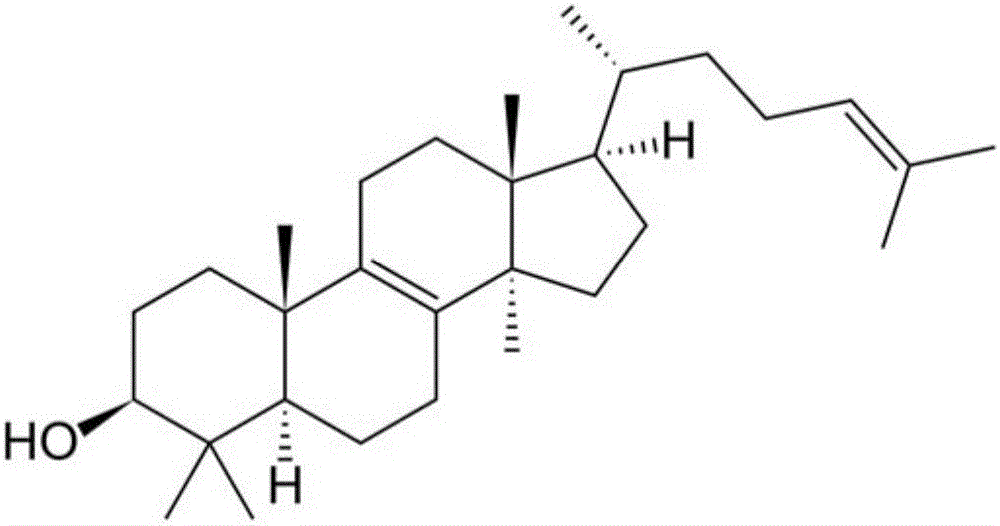
Lanosterol compound preparation for eyes
An ophthalmic preparation, technology of lanosterol, applied in the field of treatment or prevention of cataracts, ophthalmic preparations of lanosterol compounds, which can solve problems such as difficult to cure cataracts, difficult to dissolve in water, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0141] Preparation of Ophthalmic Preparations
[0142] The ophthalmic preparation of the present invention can be prepared with conventional equipment and methods according to the pharmaceutical components and proportions provided by the method of the present invention. Including the following methods:
[0143] Method 1: (a) a pharmaceutically acceptable carrier; and (b) a lanosterol compound as the first active ingredient are mixed to form the ophthalmic preparation of the present invention.
[0144] Method 2: mixing (a) a pharmaceutically acceptable carrier; and (b) a lanosterol compound as the first active ingredient and (c) the second active ingredient to form the ophthalmic preparation of the present invention .
[0145] Method 3:
[0146] (i) dispersing the lanosterol compound as the first active ingredient and the optional second active ingredient in the polyol to form a first dispersion;
[0147] (ii) mixing the first dispersion with the rest of the pharmaceuticall...
Embodiment 1
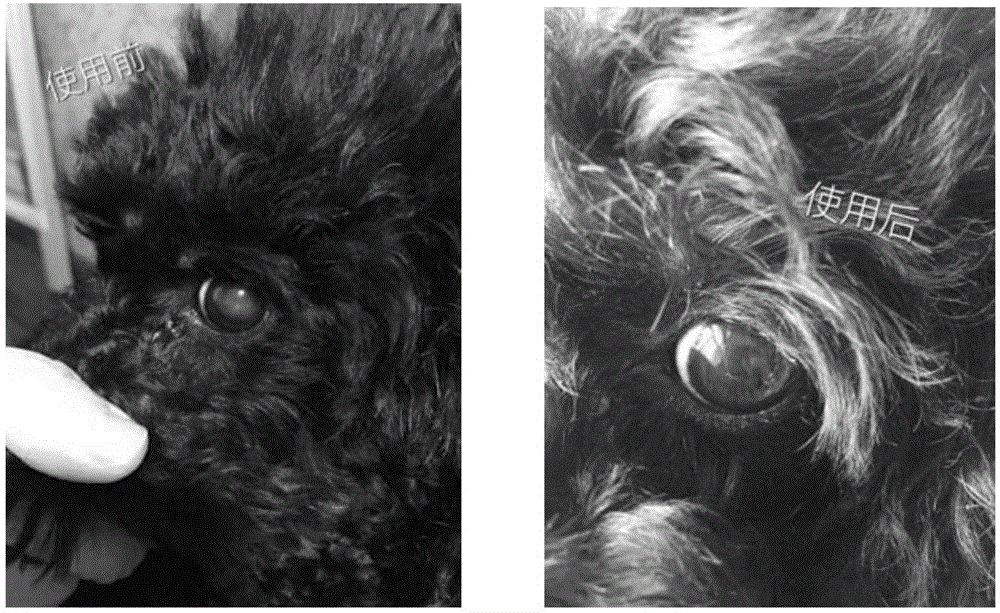
[0163] According to the literature (Zhao, Chen et al.2015), the eye drops were prepared according to the formula, and the therapeutic effect of lanosterol eye drops alone on cataract in dogs was evaluated without simultaneous intravitreal injection. 25mM lanosterol eye drops contain 12.5g lanosterol (Tokyo Chemical Industry, Japan), 200ml medical ethanol and 1.1g (EDTA) 2 Na and 0.55 g of benzalkonium chloride, the whole solution was prepared with triple distilled water and dissolved in 1.1 L. Incompletely dissolved lanosterol particles can be clearly seen inside the prepared solution.
[0164] The above-mentioned 25mM lanosterol eye drops are administered three times a day in the morning, noon and evening, with an interval of at least 5 hours between administrations. The subjects of the administration were dogs with cataract caused by various reasons. A total of 7 cataract-affected eyes participated in the test. The administration method was to drop directly into the eyes to...
Embodiment 2
[0166] The eye drop of the present invention is uniform, non-suspended, white liquid, completely water phase, the cosolvent is cyclodextrin, preferably hydroxypropyl-β-cyclodextrin, the concentration of lanosterol is 25mM, and the whole eye drop has no See white particles that are insoluble in the aqueous phase.
[0167] Eye Drops Formula:
[0168]
[0169] Note: During the preparation process, physical dissolution methods such as conventional ultrasound and heating can be used.
[0170] Administration method:
[0171] The above pharmaceutical preparations are administered three times a day in the morning, noon and evening, with an interval of at least 5 hours between administrations. The administration objects are dogs with cataract caused by various reasons, and the administration method is to drop directly into the eyes to ensure that the medicine is completely instilled into the eyes. One drop, about 50 microliters, was administered to each affected eye of each treat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| osmolarity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com