Application of mannuronic acid oligose with carboxyl at 1-position of reducing end and derivative to treatment of Parkinson's disease
A technology of uronic acid oligosaccharides and derivatives, which is applied in the preparation of sugar derivatives, sugar derivatives, and sugar derivatives, and can solve the problems of drug application restrictions and large molecular weight of alginate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
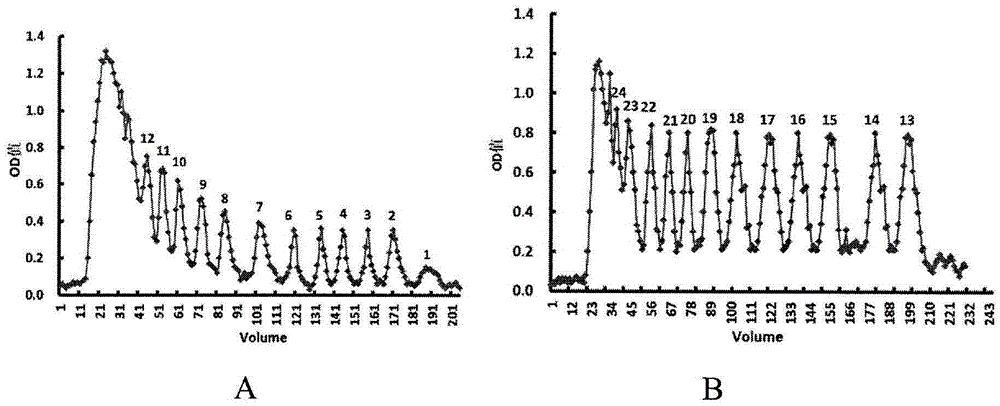
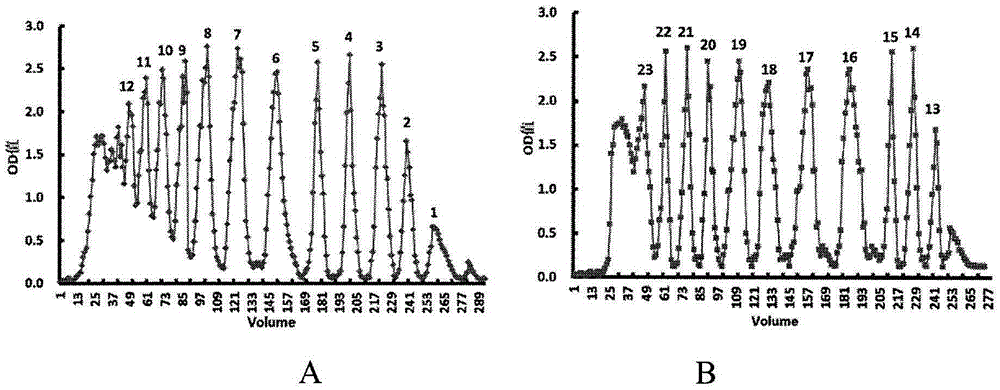
[0044] Example 1 Preparation of Alginate Oligosaccharides
[0045] Weigh 1 g of polymannuronic acid sodium salt (weight average molecular weight 8235 Da, provided by Shanghai Green Valley Pharmaceutical Co., Ltd.), and add appropriate amount of distilled water to prepare a 1% (weight percent) aqueous solution of polymannuronate sodium. The pH value of the 1% aqueous sodium polymannuronate solution was adjusted to 4 with hydrochloric acid, and then the aqueous solution was placed in an autoclave. The reaction was heated at 110°C for 4 hours. The reacted solution was taken out from the autoclave and allowed to cool. After cooling, the pH value of the reacted solution was adjusted with NaOH solution to obtain a neutral liquid. Under the condition of stirring, the neutral liquid was slowly added into ethanol in an amount 4 times the volume of the liquid, alcohol precipitation was carried out and the mixture was allowed to stand overnight. The solid matter obtained by alcohol pr...
Embodiment 2
[0047] Example 2 Preparation of algin oligosaccharide oxygen hydrolysis product
[0048] Take 5 g of the above-mentioned crude alginate oligosaccharides that have not been separated by molecular exclusion chromatography to prepare a 5% (weight percent) aqueous solution. Fresh oxidant copper hydroxide was prepared by adding 25 ml of 5% (weight percent) copper sulfate solution to 50 ml of 10% (weight percent) sodium hydroxide solution and mixing immediately. The fresh oxidant copper hydroxide was immediately added to 40ml of the above-mentioned 5% (weight percent) alginate oligosaccharide solution, and heated in a boiling water bath until no brick-red precipitate was produced. The reaction system was centrifuged to remove precipitates to obtain a supernatant. Get a little supernatant and add the oxidizing agent again to check whether there is brick red precipitate. If there is still a brick-red precipitate, then continue to react all the supernatant obtained by the centrifugat...
Embodiment 3
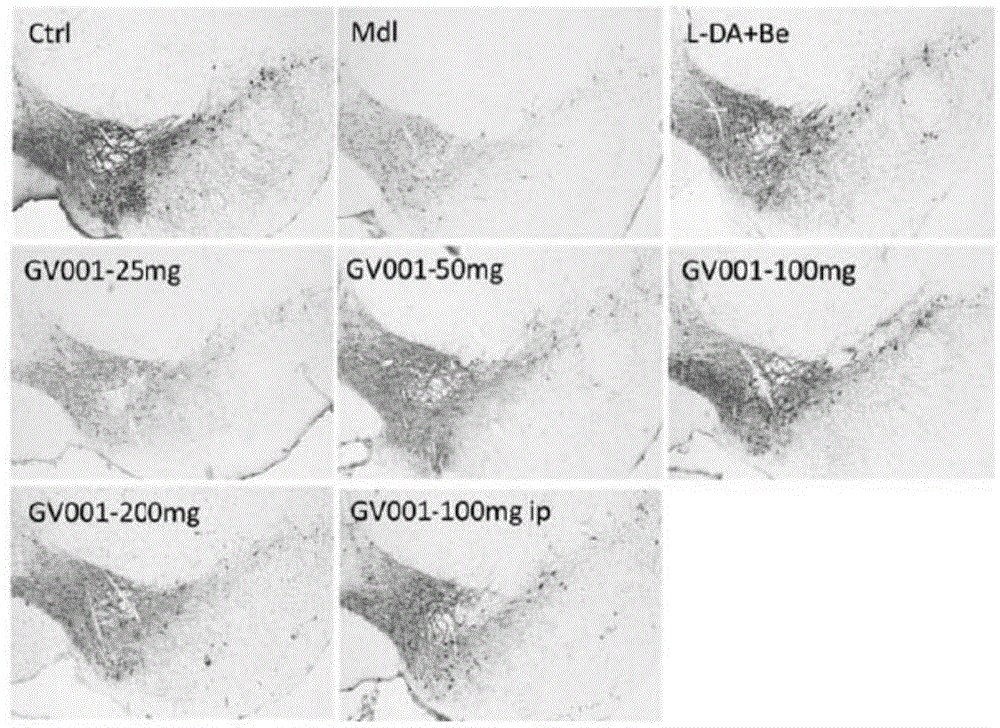
[0050] Example 3 The effect of alginate oligosaccharide derivatives in treating Parkinson's disease
[0051] 1 Experimental materials
[0052] 1.1 Experimental animals
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com