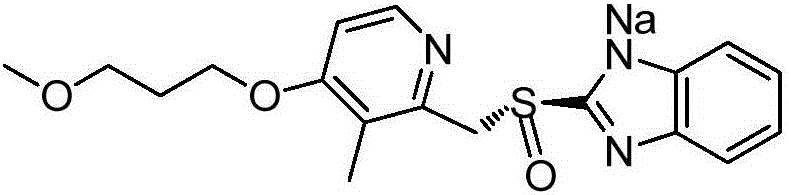
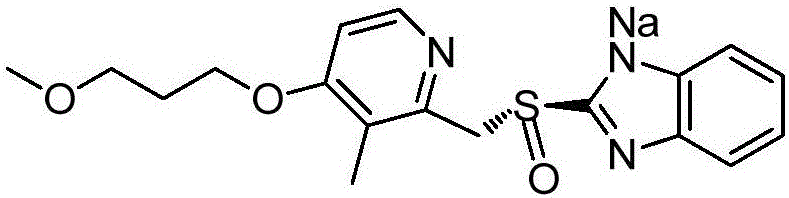
Purifying method for rabeprazole sodium
A technology of rabeprazole sodium and a purification method, which is applied in the field of medicine, can solve the problems of high quality requirements for crude dex-rabeprazole sodium, poor concentration of dex-rabeprazole sodium, long reaction time and easy oxidation, etc. To achieve the effect of overcoming impurities that are easily brought in, reducing residual dissolution, and improving the effect of clinical medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A method for purifying dexrabeprazole sodium, comprising the following steps:
[0034] (1) the dextro-rabeprazole sodium crude product is dissolved in 25% ammonia water, and toluene solvent is added;
[0035] (2) adjusting the pH of the solution obtained in step (1) to 7.5 with 50% glacial acetic acid;
[0036] (3) adding step (2) gained solution to the activated carbon through special treatment, and the consumption is 20% of the dextro-rabeprazole sodium quality;
[0037] (4) the solution obtained in step (3) was stirred for 1 hour;
[0038] (5) the solution obtained in step (4) is filtered and layered;
[0039] (6) adding an appropriate amount of NaOH aqueous solution to the solution obtained in step (5) and stirring to form a salt, then filtering to obtain a pure product with a purity of 99.9% as detected by HPLC, the single impurity does not exceed 0.1%, and the total impurity does not exceed 0.5%.
Embodiment 2
[0041] A method for purifying dexrabeprazole sodium, comprising the following steps:
[0042] (1) dextro-rabeprazole sodium crude product is dissolved in 12.5% ammonia water, and toluene solvent is added;
[0043] (2) adjusting the pH of the solution obtained in step (1) to 8.5 with 50% glacial acetic acid;
[0044] (3) adding step (2) gained solution to the activated carbon through special treatment, and the consumption is 25% of the dextro-rabeprazole sodium quality;
[0045] (4) the solution obtained in step (3) was stirred for 1.5 hours;
[0046] (5) the solution obtained in step (4) is filtered and layered;
[0047] (6) The solution obtained in step (5) is added with an appropriate amount of NaOH aqueous solution, stirred into a salt, and filtered to obtain a pure product. The purity detected by HPLC is 99.8%. .
Embodiment 3
[0049] A method for purifying dexrabeprazole sodium, comprising the following steps:
[0050] (1) dextro-rabeprazole sodium crude product is dissolved in 12.5% ammonia water, and toluene solvent is added;
[0051] (2) adjusting the pH of the solution obtained in step (1) to 7.5 with 50% glacial acetic acid;
[0052] (3) adding step (2) gained solution to the activated carbon through special treatment, and the consumption is 25% of the dextro-rabeprazole sodium quality;
[0053] (4) the solution obtained in step (3) was stirred for 1 hour;
[0054] (5) the solution obtained in step (4) is filtered and layered;
[0055] (6) adding an appropriate amount of NaOH aqueous solution to the solution obtained in step (5) and stirring to form a salt, then filtering to obtain a pure product with a purity of 99.9% as detected by HPLC, the single impurity does not exceed 0.1%, and the total impurity does not exceed 0.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com