A quality evaluation method for Qianliexin capsules based on the determination of multi-index active ingredients
A technology for active ingredient and quality evaluation, applied in the field of pharmaceutical testing, can solve the problems of not being able to fully reflect the multi-component and multi-target of traditional Chinese medicine prescriptions, unable to meet the quality control of finished drugs, unable to reflect the overall quality of the drug, etc. Guaranteed effectiveness and safety, the effect of more comprehensive analysis capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
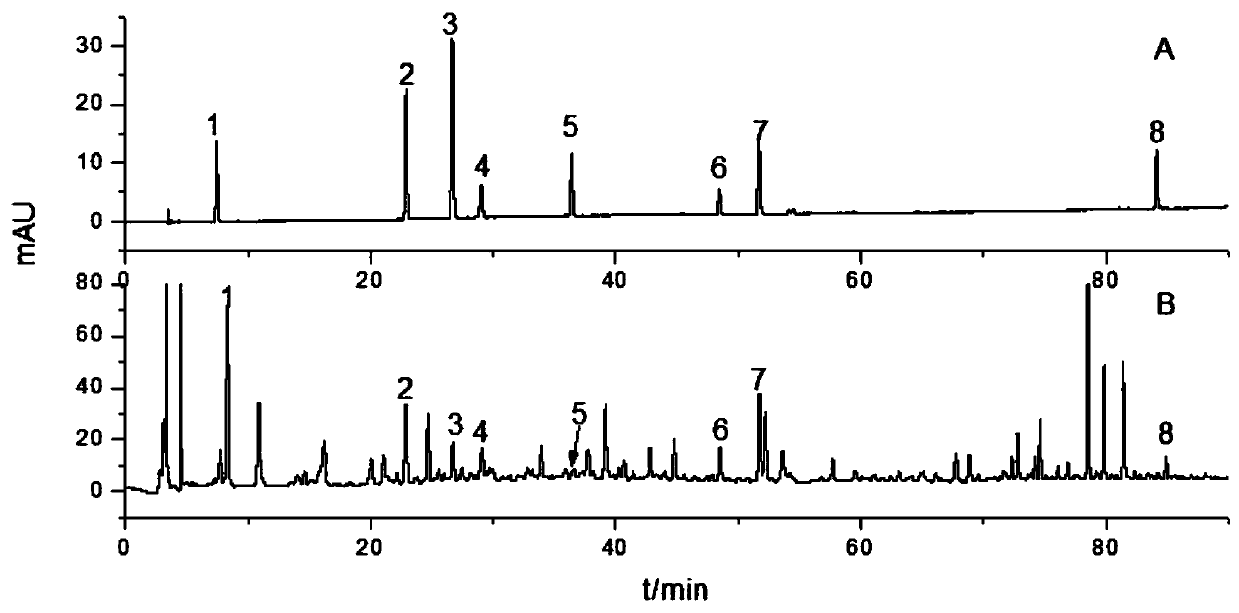
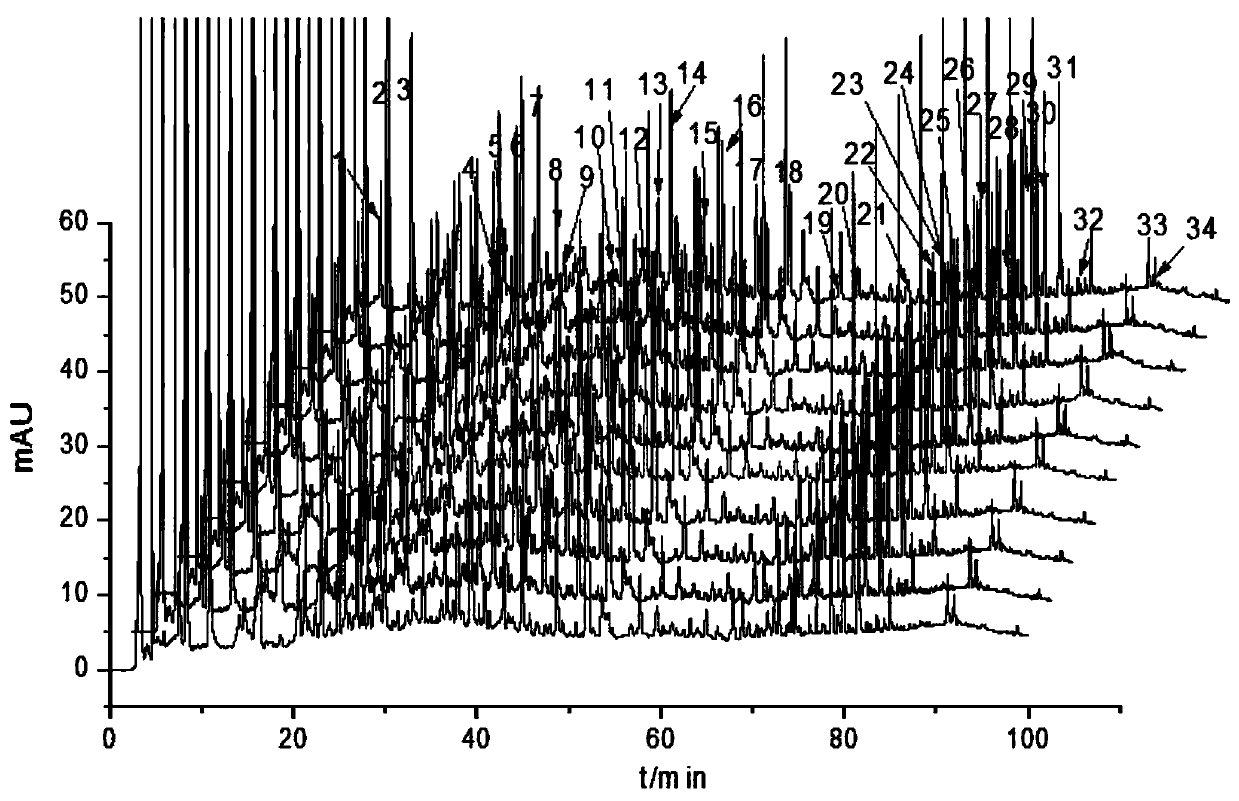
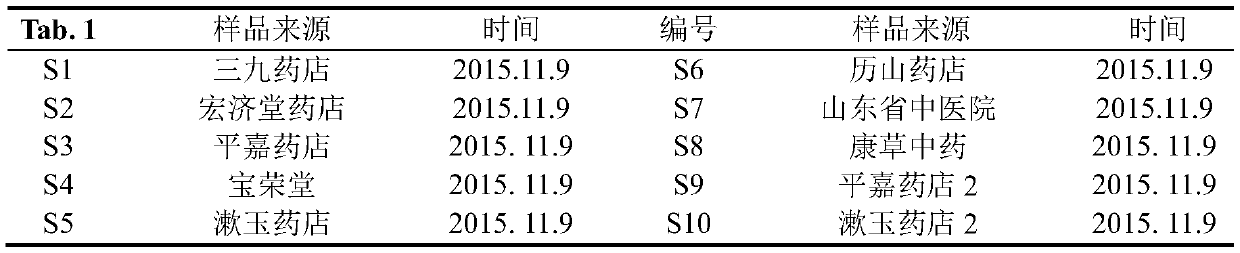
[0032] 1 Instruments and materials
[0033] Agilent 1260 high performance liquid chromatograph (autosampler, gradient pump, column thermostat, diode array detector); one hundred thousandth electronic analytical balance (SARTOURIUS BSA), fingerprint similarity evaluation software is "Chinese medicine chromatographic fingerprint Similarity Evaluation System" (National Pharmacopoeia Commission 2004A); SB-5200D high-power digitally controlled ultrasonic instrument (Ningbo Xinzhi Biotechnology Co., Ltd.).
[0034] Column: Agilent ZORBAX SB-C, USA 18 (4.6mm×250mm, 5μm), Kromasil 100-5C, Sweden 18 (4.6mm×250mm, 5μm). Gallic acid (batch number 149-91-7), chlorogenic acid (batch number 327-97-9), caffeic acid (batch number 331-39-5), Wangbuliuxing flavone glycosides (batch number 53452-16-7), iso Quercetin (lot number 482-35-9), salvianolic acid B (lot number 115939-25-8), salvianolic acid A (lot number 96574-01-5), cryptotanshinone (lot number 35825-57-1), standard The purity of the prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com