Iron-based catalyst used in preparation of low-carbon olefin from synthetic gas, and preparation method and application thereof
A technology for iron-based catalysts and low-carbon olefins, which is applied in catalyst activation/preparation, carbon compound catalysts, catalysts, etc., can solve problems to be further improved, and achieves favorable conditions for large-scale production and application, low energy consumption, and preparation conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
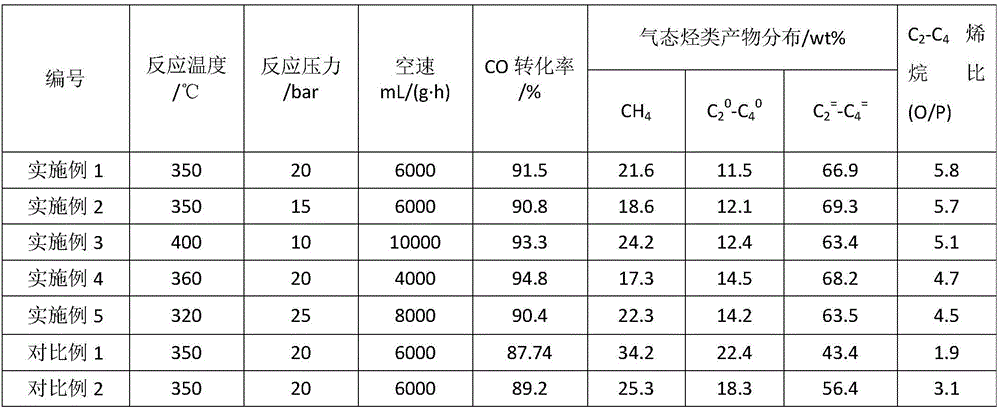
Embodiment 1
[0021] Weigh 73.6g of aluminum nitrate and 29.4g of urea, dissolve them in 20mL of distilled water and place them in a water bath at 60°C to heat and stir evenly, then pour the solution into a ceramic evaporating dish and place it in an air atmosphere in a muffle furnace at 500°C Spontaneously ignite after heating, and continue to maintain for 20 minutes to take out the solid product after combustion, and grind the product after cooling to obtain powder α-Al with a mesh size greater than 200 mesh 2 o 3 About 10g.
[0022] Weigh 4.1g of ferric nitrate, 0.21g of sodium nitrate and 1.5g of urea and dissolve in 2.5mL of distilled water to obtain a mixed solution, and weigh the prepared α-Al 2 o 3 5g of the carrier was slowly added into the above mixed solution under stirring condition and dipped and stirred for 12 hours for later use. Pour the impregnation solution prepared above into a ceramic evaporating dish, place it in an air atmosphere in a muffle furnace and heat it at 4...
Embodiment 2
[0026] Weigh 73.6g of aluminum nitrate and 29.4g of urea, dissolve them in 20mL of distilled water, put them in a water bath, heat and stir at 60°C, then pour the solution into a ceramic evaporating dish, place it in an air atmosphere in a muffle furnace, and heat at 450°C Spontaneously ignite and keep for 20 minutes, take out the solid product after cooling, and grind the product after cooling to obtain a powder α-Al with a mesh size greater than 200 mesh 2 o 3 About 10g.
[0027] Weigh 4.1g of ferric nitrate, 0.15g of potassium nitrate and 1.5g of urea, add 2.5mL of distilled water to dissolve to obtain a mixed solution, weigh the prepared catalyst carrier α-Al 2 o 3 5g, slowly added to the above mixed solution under stirring condition and soaked and stirred for 12h for later use. Pour the above-prepared impregnating liquid into a ceramic evaporating dish, place it in an air atmosphere in a muffle furnace and heat it at 480°C to spontaneously ignite, and keep it for 20 m...
Embodiment 3
[0030] Weigh 73.6g of aluminum nitrate and 29.4g of urea, dissolve them in 20mL of distilled water, put them in a water bath, heat and stir at 60°C, then pour the solution into a ceramic evaporating dish, place it in an air atmosphere in a muffle furnace, and heat at 550°C Spontaneously ignite and keep for 20 minutes, take out the solid product after cooling, and grind the product after cooling to obtain a powder α-Al with a mesh size greater than 200 mesh 2 o 3 About 10g.
[0031] Weigh 6.5g of ferric nitrate, 0.15g of potassium nitrate, 0.11g of sodium nitrate and 2.6g of citric acid, add 4.5mL of distilled water to dissolve to obtain a mixed solution, weigh the prepared catalyst carrier α-Al 2 o 3 5g, slowly added to the above mixed solution under stirring condition and soaked and stirred for 12h for later use. Pour the impregnation solution prepared above into a ceramic evaporating dish, place it in an air atmosphere in a muffle furnace and heat it at 400°C to spontane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com