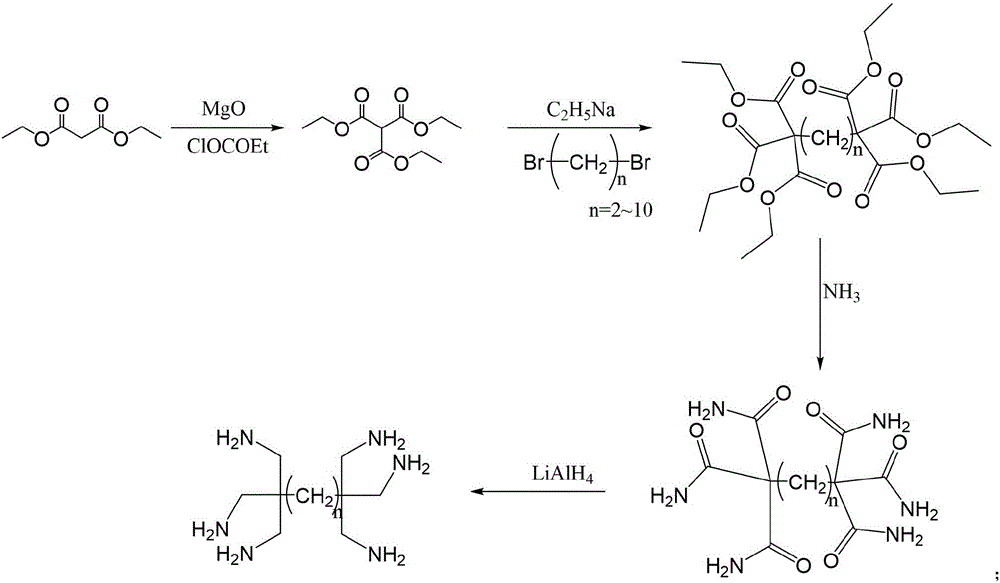
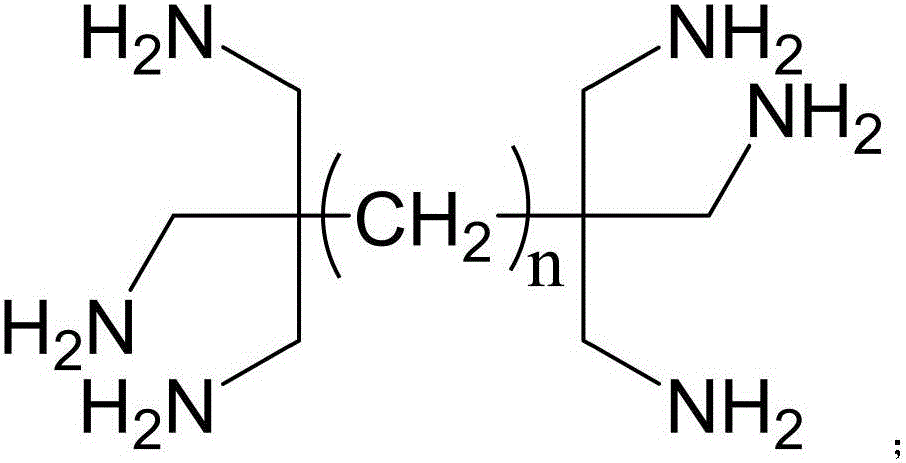
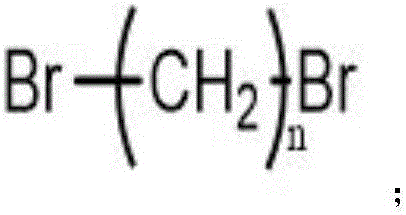Ultralow molecular weight dendritic alkyl hexamine shale inhibitor and synthetic method thereof
A technology of an alkylhexamine and a synthesis method is applied in the synthesis of dendritic alkylhexamine shale inhibitors and the field of shale inhibitors prepared from alkylhexamines, achieving high yield, stable performance and high price. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Adopt the following ratio and steps to synthesize alkyltetramines, the steps are as follows:
[0046] Synthesis of S1, triethyl methanetricarboxylate
[0047] S1.1. Add 0.4mol of magnesium oxide, 50ml of dry toluene, 0.25mol of diethyl malonate and 0.20mol of ethyl chloroformate to a dry 250ml three-necked flask, and mix well;
[0048] S1.2. The mixture was refluxed and reacted at 75°C for 7 hours; after the reaction was completed, cool to 5°C, and add pure water at room temperature dropwise until all the precipitates became granular or paste-shaped cakes;
[0049] S1.3. Gently decant the solution, wash the precipitated part with 15ml ether and filter; carefully neutralize the filtered precipitated part with 5mol / L hydrochloric acid under ice bath conditions; after the solid dissolves, use 40ml×2 Extract with water and ether, and combine the organic phases;
[0050] S1.4, the organic phase was washed with 1mol / L hydrochloric acid, water, 5% sodium bicarbonate, and bri...
Embodiment 2
[0064] Synthesis of S1, triethyl methanetricarboxylate
[0065] S1.1. Add 0.6 mol of magnesium oxide, 50 ml of dry toluene, 0.28 mol of diethyl malonate and 0.20 mol of ethyl chloroformate in a dry 250 ml three-necked flask, and mix well;
[0066] S1.2. The mixture was refluxed and reacted at 90°C for 7 hours; after the reaction was completed, cool to 5°C, and add pure water at room temperature dropwise until all the precipitates became granular or paste-shaped cakes;
[0067] S1.3. Gently decant the solution, wash the precipitated part with 15ml ether and filter; carefully neutralize the filtered precipitated part with 5mol / L hydrochloric acid under ice bath conditions; after the solid dissolves, use 40ml×2 Extract with water and ether, and combine the organic phases;
[0068] S1.4, the organic phase was washed with 1mol / L hydrochloric acid, water, 5% sodium bicarbonate, and brine, and finally dried with anhydrous sodium sulfate; diethyl ether was evaporated to obtain a trie...
Embodiment 3
[0086] Synthesis of S1, triethyl methanetricarboxylate
[0087]Add 0.5 mol of magnesium oxide, 50 ml of dry toluene, 0.26 mol of diethyl malonate and 0.20 mol of ethyl chloroformate into a dry 250 ml three-necked flask in sequence, and mix well; the mixture is refluxed at 80°C 7h; after the reaction is completed, cool to 5°C, add pure water at room temperature drop by drop until all the precipitates become granular or paste-shaped cakes; pour off the solution gently, wash the precipitated part with 15ml ether and filter; after filtration The precipitated part was carefully neutralized with 5mol / L hydrochloric acid under ice bath conditions; after the solid was dissolved, it was extracted with anhydrous ether, and the organic phase was combined; the organic phase was respectively treated with 1mol / L hydrochloric acid, water, and 5% hydrogen Sodium, washed with brine, and finally dried with anhydrous sodium sulfate; evaporated ether to obtain the product triethyl methanetricarbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com