Function and application of IRF5 (interferon regulatory factor-5) and IRF5 inhibitor in treatment of restenosis after VI (vascular injury)
A technology for vascular injury and restenosis, applied in gene therapy, cardiovascular system diseases, blood diseases, etc., can solve problems such as high incidence and affecting treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] [Example 1] Mouse vascular injury model (VI) obtained
[0045] 1. Grouping of experimental animals: WT and IRF5-KO mice aged 8-10 weeks and weighing 24-27 g were used, and were divided into two groups: WT vascular injury group and IRF5-KO vascular injury group, with 20 mice in each group. mouse. The mice were sacrificed 14 days and 28 days after the operation, and blood vessels in the injured segment were collected for analysis.
[0046] 2. Operation procedure of mouse vascular injury model:
[0047] 1) Accurately weigh the body weight (g) of the mouse in dynamic mode with an electronic balance, accurately prepare 3% pentobarbital sodium solution with double distilled water, shake gently to dissolve it fully, and use 80 mg / kg body weight dose to calculate Accurately extract the corresponding volume of solution with a 1mL syringe after the required volume of pentobarbital sodium solution, and perform intraperitoneal injection to anesthetize the mouse. After the mouse i...
Embodiment 2
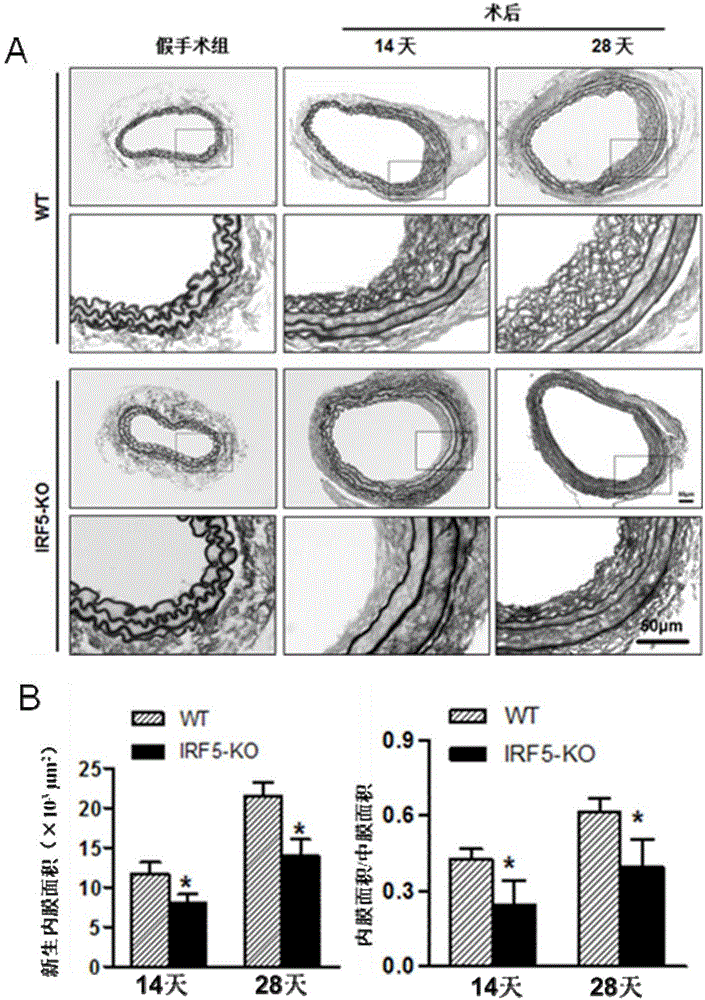
[0052] [Example 2] Intimal Neonatal Determination in Vascular Injury Model (VI) Mice
[0053] 1. Mice collection
[0054] 1) Anesthetize the mouse, cut the heart and let the blood out.
[0055] 2) Cut the carotid artery from the proximal bifurcation of the carotid artery, take 0.5-0.6cm long, and keep the external carotid artery knot.
[0056] 3) Put the carotid artery into PBS, and gently drain the residual blood in the lumen with micro forceps.
[0057] 4) Put the blood vessel into a 1.5mL EP tube filled with 1mL 4% paraformaldehyde for fixation.
[0058] 2. Pathological detection
[0059] 2. Pathological detection
[0060] 2.1 Preparation of paraffin specimen slices
[0061] Paraffin specimen slices are prepared by professional pathologists in the laboratory. The main operating procedures include: after overnight fixation in 4% paraformaldehyde, carefully wrap the blood vessel with filter paper, put it into the embedding frame→rinse with running water→dehydration→trans...
Embodiment 3
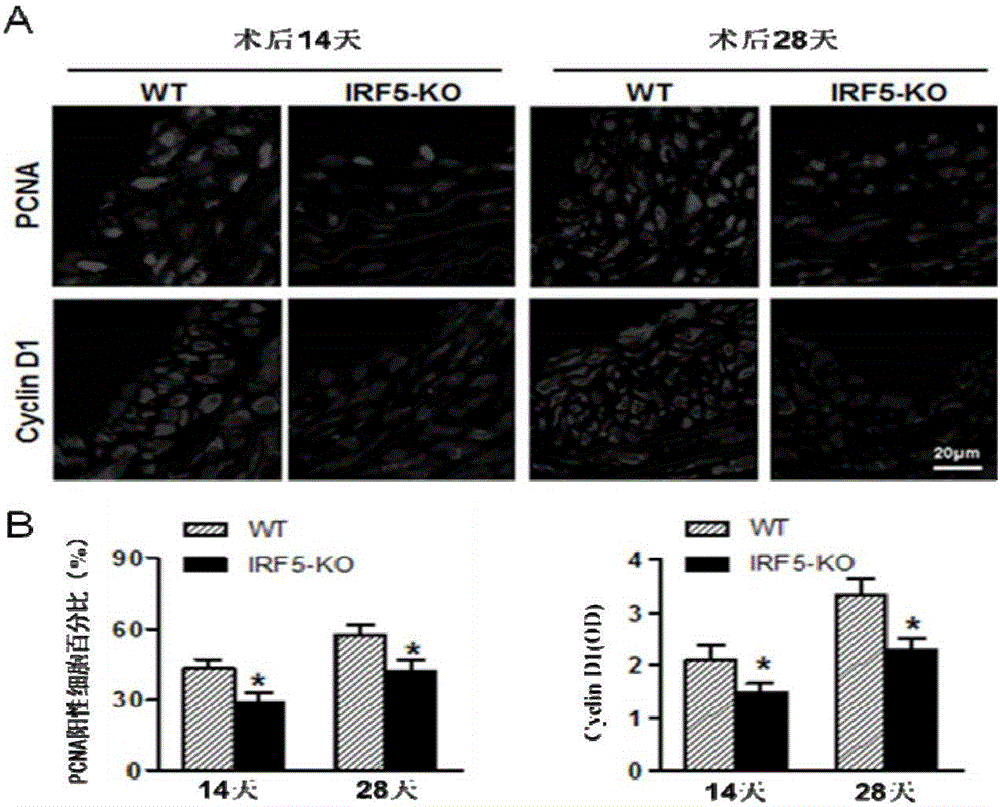
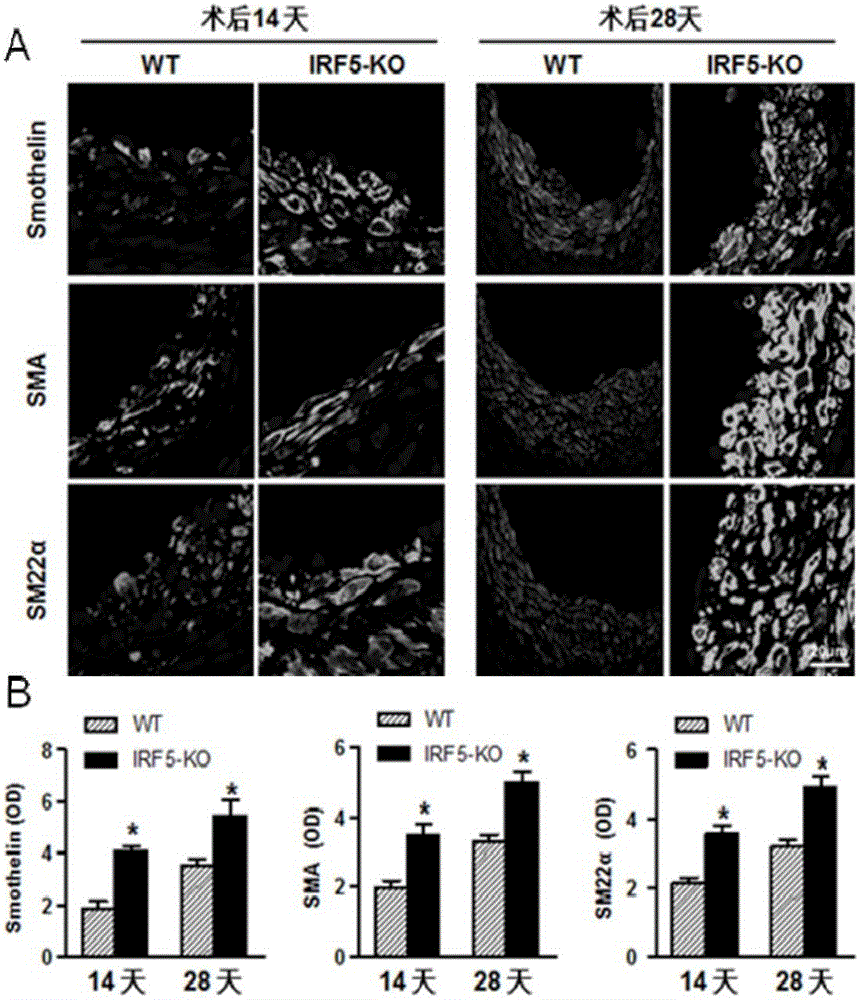
[0069] [Example 3] Detection of the proliferation level of blood vessel wall cells
[0070] Immunofluorescent staining was used to detect the expression of proliferating cell nuclear antigen (PCNA) and cell cycle protein (Cyclin D1). Required primary antibody information: PCNA (#2586; 1:100; mouse; Cell Signaling Technology), cyclin D1 (#2978; 1:25; rabbit; Cell Signaling Technology); required secondary antibody information: Alexa Fluor 568-conjugated goat anti-rabbit IgG (A11011; Invitrogen, Carlsbad, CA), Alexa Fluor 568-conjugated goat anti-mouse IgG (A11004; Invitrogen, Carlsbad, 150d, CA).
[0071] The main steps are:
[0072] 1) Baked slices: put the paraffin slices in an oven at 55°C for more than 60 minutes.
[0073] 2) Dewaxing: xylene 8min×3.
[0074] 3) Hydration: 100% ethanol 5min×2; 95% ethanol 5min; 70% ethanol 5min; ddH 2 O dipping for 5min×2.
[0075] 4) Citrate tissue antigen repair (high pressure repair): Take a certain amount of pH6.0 citrate antigen repa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com