New carboxyl terminal peptide and long-acting interferon
A carboxy terminal peptide, interferon technology, applied in the direction of interferon, cytokine/lymphokine/interferon, peptide, etc., can solve the problems of enhancing the biological activity of fusion protein, prolonging half-life, reducing renal clearance rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1 material
[0053] 1.1 Main reagents and materials
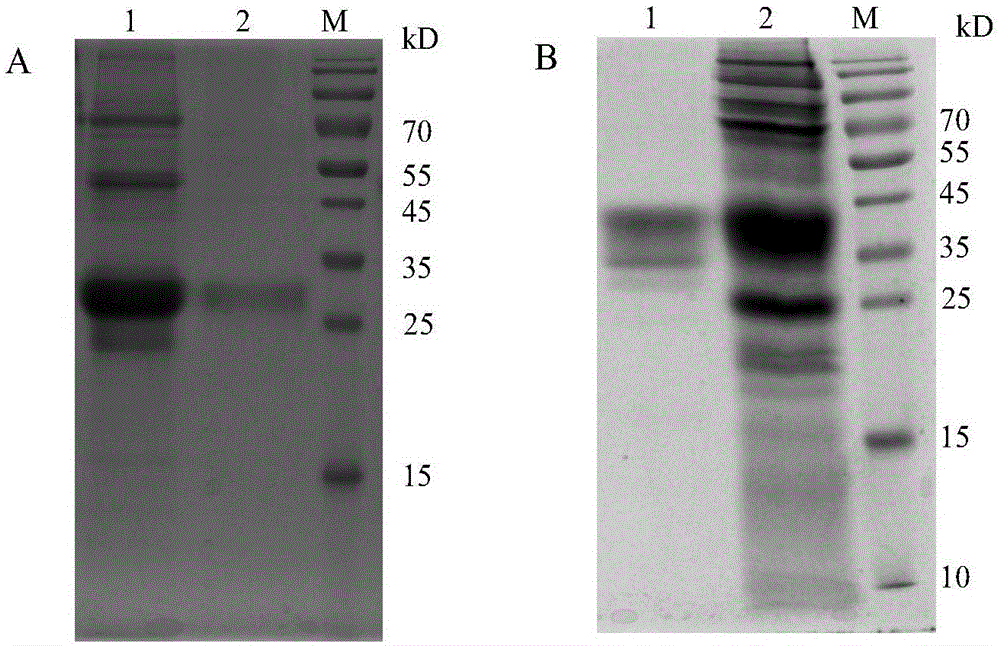
[0054] The complete sequence of rhIFN-λ1 gene (AY184372) and CTP sequence were synthesized by Invitrogen (USA). Competent Escherichia coli E. Coli strain DH5α was purchased from Tiangen Biological Company. The cloning vector pMD18T was purchased from Takara Company. T4 DNA ligase is a Promega product. Flp-In-CHO cells, pOG44 plasmid and pcDNA5 / FRT plasmid were purchased from Invitrogen (USA). rhIFN-λ1 standard protein was purchased from R&D Company; IL-29Human ELISA Kit was purchased from Abcam Company. Taq DNA polymerase, restriction endonuclease NheI / HindIII, etc. were purchased from Takara Company. Protein purification gel SP Sepharose FF, Blue Sepharose6, S-100 were purchased from GE Company of the United States. TRIZOL, RT-PCR Kit, SⅢ reverse transcription kit was purchased from Invitrogen; Qiagen RNeasy protect min kit and RNasefree DNase set were purchased from Qiagen; Cytotox96non-radioactive Cytotoxi...
Embodiment 2
[0077] Embodiment 2 long-acting determination
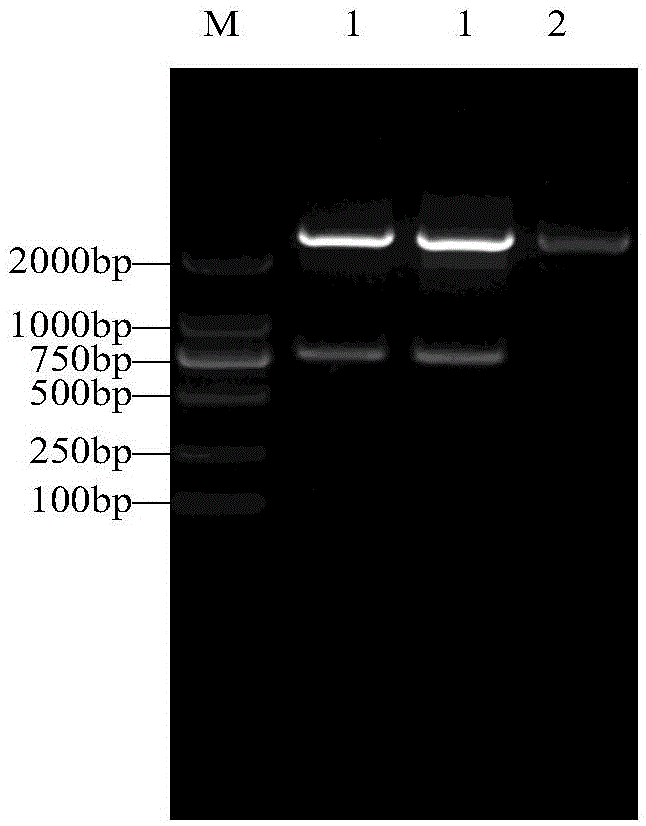
[0078] The rhIFN-λ1-CTP without N-glycosylation site was prepared according to the method of Example 1. First, the target fragment was obtained by PCR, the upstream primer was the same, and the downstream primer SEQ ID NO: 6 was aagctt ttatcattgtgggaggatcggggtgtccga, after connecting the pDF vector, it was verified by restriction restriction sequencing, and the rhIFN-λ1-CTP protein was purified by transfection and screening to obtain a stable clone by the same method as in Example 1.
[0079] Balb / c mice were divided into three groups, 6 mice in each group, and were given intraperitoneal injection of rhIFN-λ1, rhIFN-λ1-CTP and rhIFN-λ1-CTPON (40 μg / kg), at 0.5, 1, 4, 8 and 24 Blood was collected every hour, and serum was obtained after centrifugation, and ELISA (IL-29 Human ELISA Kit, Abcam Company) kit was used to detect the content of each sample in the serum. The results showed that the concentration of rhIFN-λ1 was only 77p...
Embodiment 3
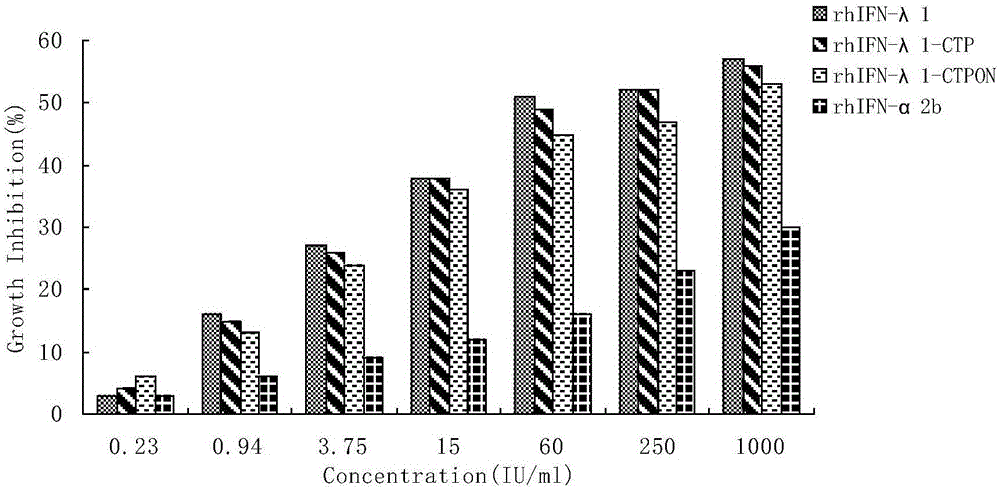
[0080] Example 3 sample antiviral activity assay:
[0081] The WISH cells growing adherently in the cell flask were digested with 0.25% trypsin and removed, and prepared with complete culture medium containing 10% fetal bovine serum to contain 4×10 5 The cell suspension of 10 cells was seeded in 96-well cell culture plate, 100 μL per well, at 37°C, 5% CO 2overnight culture under conditions. The next day, the prepared rhIFN-λ1, rhIFN-λ1-CTPON and rhIFN-λ1 purchased by R&D Company and 1000IU / mL IFNα-2b standard (4 0 -4 9 gradient dilution), add 100 μL of diluted interferon sample to each well, and duplicate wells for each gradient. At 37°C, 5% CO 2 Cultivate under conditions for 6-12 hours, discard the supernatant in the cell culture plate, dilute the preserved VSV virus to about 100 TCID50 with DMEM culture solution containing 2% fetal bovine serum, and add 150 μL to each well. Then at 37°C, 5% CO 2 Cultivate for 24 hours (50% of the lesion points of the microscopic exami...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com