Lactobacillus probiotics CGMCC NO. 12422 and application of lactobacillus probiotics in preparing lipid-lowering drug
A technology of CGMCCNO.12422, Lactobacillus, which is applied in the field of microorganisms, can solve the problems that lactic acid bacteria cannot achieve satisfactory cholesterol-lowering effects, lactic acid bacteria screening indicators, in vivo effects of regulating blood lipids, and unclear mechanisms.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
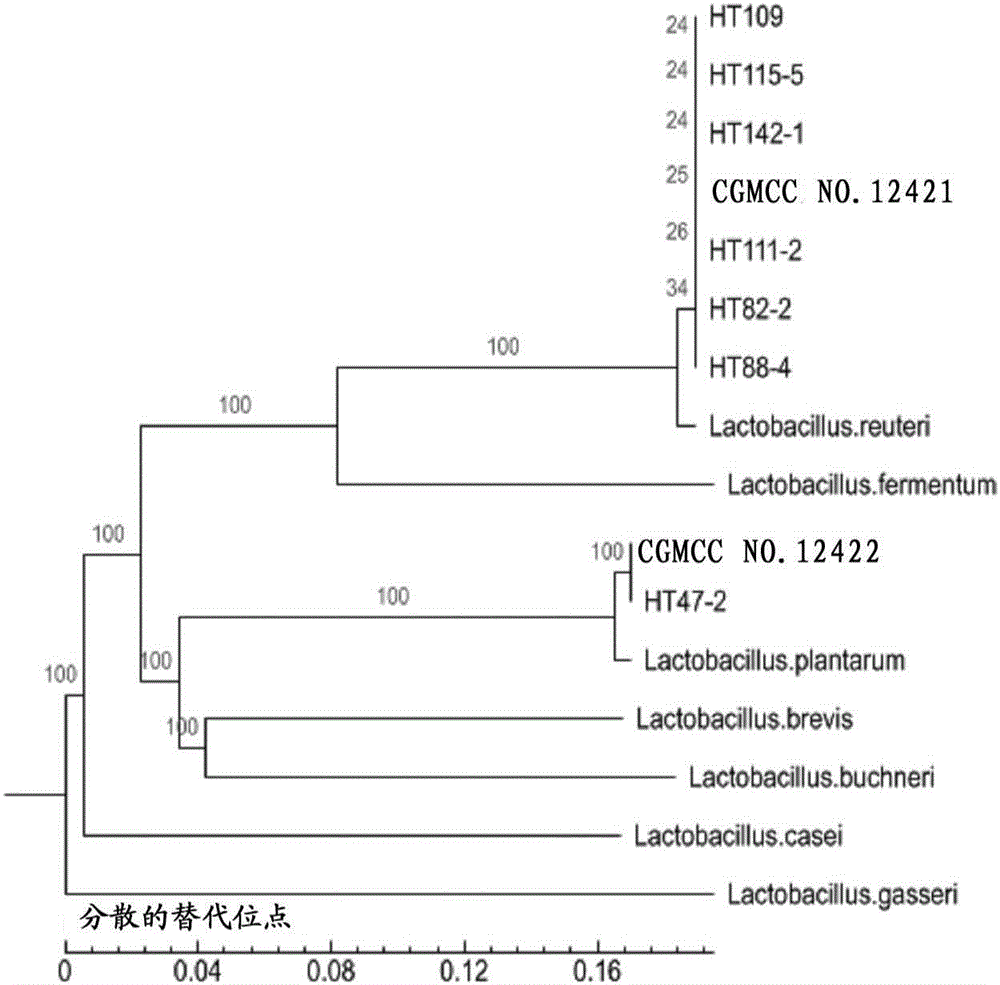
[0017] Embodiment 1. Isolation, screening and identification of lactic acid bacteria
[0018] 1. Isolation of Lactobacillus
[0019] 1) Take 100 μL of the sample from the sterile tube, add it to the EP tube pre-filled with 900 μL sterile PBS, and sequentially dilute the sample, and dilute the concentration of the marmot feces sample to 10 -6 times;
[0020] 2) Take 100 μL of samples with different dilutions and spread them on the MRS medium, and put them into the incubator;
[0021] 3) At 37°C, 0.5% CO 2 Cultivate in the environment for 48h;
[0022] 4) Take out the petri dish, use a sterile inoculation loop to pick colonies with different morphological characteristics, transfer them to a new MRS solid medium for purification, culture anaerobically at 37°C for 48 hours, transfer 3 times continuously, and transfer the purified strains at pH =3.5 of the liquid MRS culture, screening acid-resistant growth of excellent strains can be used for experiments or cryopreservation. ...
Embodiment 2
[0058] Embodiment two, lipid-lowering function evaluation of lactic acid bacteria
[0059] 1. In vitro evaluation of lipid-lowering function
[0060] (1) Configuration of cholesterol-containing medium
[0061] 1) MRS medium containing 0.1% bile salt: weigh bile salt powder at 0.1%, add it to 400ml prepared MRS liquid medium, mix thoroughly, and then sterilize by high pressure steam at 121°C for 15 minutes for use.
[0062] 2) MRS-CHOL (high cholesterol MRS medium): Accurately weigh 0.5g of cholesterol, dissolve it with a small amount of absolute ethanol and dilute to 50mL with a final concentration of 10.0mg / mL, filter through a microporous membrane with a pore size of 0.22μm After sterilization, add 5% into the sterilized MRS liquid medium (or MRS liquid medium containing 0.1% bile salt), so that the final concentration of cholesterol in the MRS liquid medium is 500 μg / mL.
[0063] (2) DAOS method to detect cholesterol content
[0064] 1) Principle
[0065] Hydrogen perox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com