All-solid-state composite polymer electrolyte and preparation method thereof
A solid polymer and polymer technology, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of unsatisfactory practicality and poor mechanical strength, and achieve good mechanical properties, reasonable structure ratio, and high conductivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
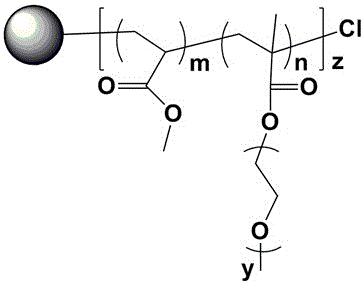
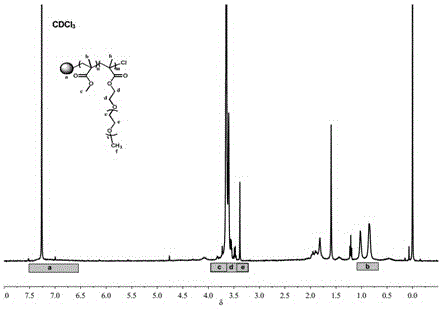
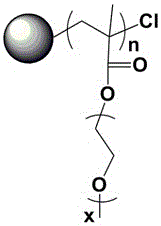
[0047] see figure 1 , 2 , 5 Dry the lithium salt in a vacuum oven at 80°C for 24 hours, weigh HBPS-(PMMA- b -PEGMA) 30(hyperbranched polystyrene is the core, methyl methacrylate-polyethylene glycol methacrylate block copolymer is the hyperbranched star polymer of the arm) 0.1g, two trifluoromethanesulfonimide lithium ( LiTFSI) 0.011g, methylimidazole tetrafluoroborate (MeImBF 4 ) 0.03g of ionic liquid, add 5ml of tetrahydrofuran solution and stir vigorously to a homogeneous solution, pour the solution into a polytetrafluoroethylene abrasive tool, evaporate the solvent at room temperature for 3h, and then place it at 60°C for 6h to obtain a polymer electrolyte. The glass transition temperature of the prepared polymer electrolyte is -57.43 ℃, and the room temperature conductivity of the polymer electrolyte is 1.99×10 -4 S / cm.
Embodiment 2
[0049] see figure 1 , 2 , 5, dry lithium salt in 80 ℃ vacuum oven for 24 hours, weigh HBPS-(PMMA- b -PEGMA) 30 (hyperbranched polystyrene is the core, methyl methacrylate-polyethylene glycol methacrylate block copolymer is the hyperbranched star polymer of the arm) 0.1g, two trifluoromethanesulfonimide lithium ( LiTFSI) 0.011g, 1-butyl-3-methylimidazole hexafluorophosphate (MeImPF 6 ) 0.03g of ionic liquid, add 5ml of tetrahydrofuran solution and stir vigorously to a homogeneous solution, pour the solution into a polytetrafluoroethylene abrasive tool, evaporate the solvent at room temperature for 3h, and then place it at 60°C for 6h to obtain a polymer electrolyte. The glass transition temperature of the prepared polymer electrolyte is -65.85 ℃, and the room temperature conductivity of the polymer electrolyte is 4.13×10 -5 S / cm.
Embodiment 3
[0051] Dry the lithium salt in a vacuum oven at 80 °C for 24 hours, weigh HBPS-(PMMA- b -PEGMA) 30 (hyperbranched polystyrene is the core, methyl methacrylate-polyethylene glycol methacrylate block copolymer is the hyperbranched star polymer of the arm) 0.1g, two trifluoromethanesulfonimide lithium ( LiTFSI) 0.011g, nano silicon dioxide 0.001g, add 5ml tetrahydrofuran solution and stir vigorously to a homogeneous solution, pour the solution into a polytetrafluoroethylene abrasive tool, evaporate the solvent at room temperature for 3h, and then place it at 60°C for 6h in vacuum to obtain polymer electrolyte. The glass transition temperature of the prepared polymer electrolyte is -48.69 ℃, and the room temperature conductivity of the polymer electrolyte is 2.53×10 -5 S / cm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Conductivity at room temperature | aaaaa | aaaaa |
| Conductivity at room temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com