A kind of preparation method of S-1 composition
A composition and technology of tegafur, applied in the direction of drug combination, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc., can solve complex cyclodextrin inclusion process, complicated production process, and strengthen drug side effects and other problems, to achieve the effects of rapid and complete drug dissolution, reduced contact degree, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 5
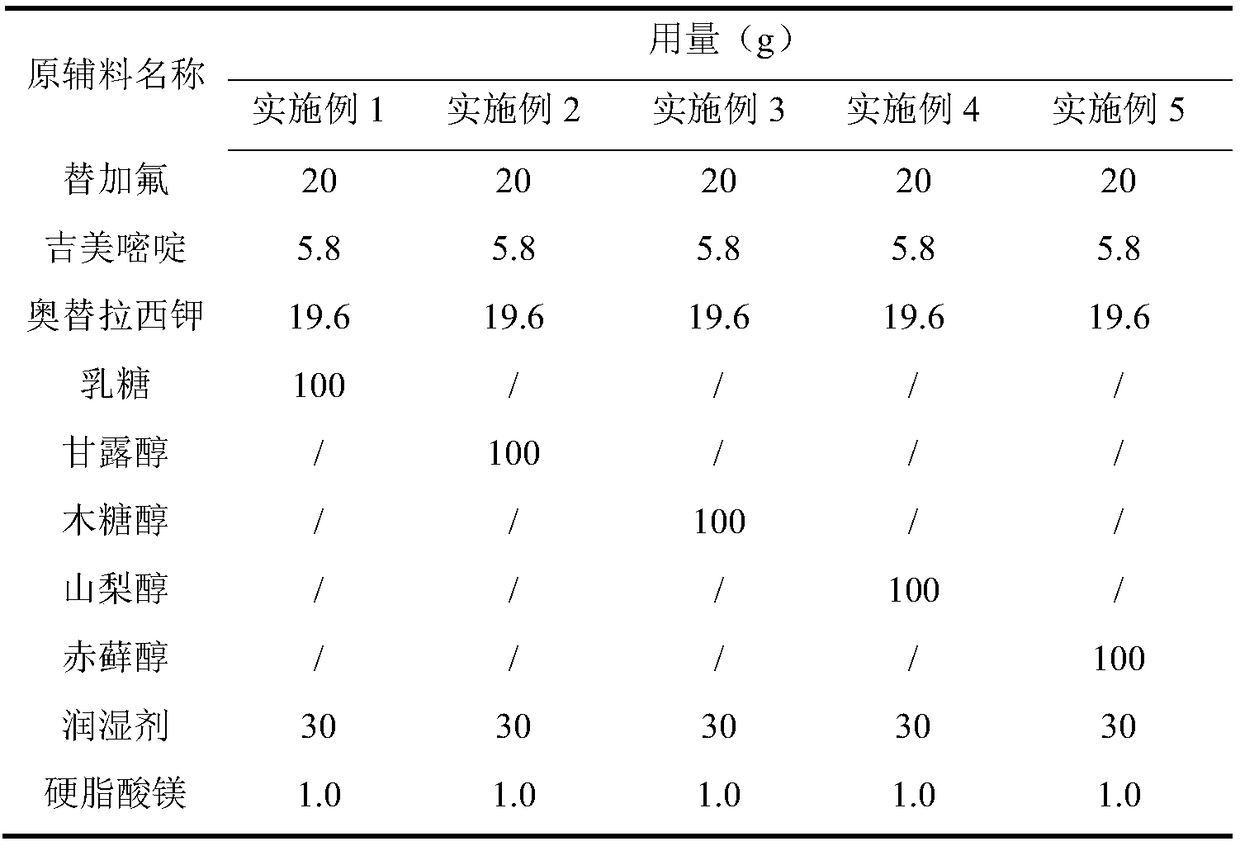
[0036] Preparation process: According to the ratio in Table 1, pass the raw and auxiliary materials through a 80-mesh sieve, mix the three raw materials evenly, add purified water, use a Glatt wet granulator to granulate, dry at 60°C, sieve through a 30-mesh sieve, and add filling agent and magnesium stearate, mixed uniformly, and filled into capsules to prepare S-1 capsules.
[0037] Table 1
[0038]
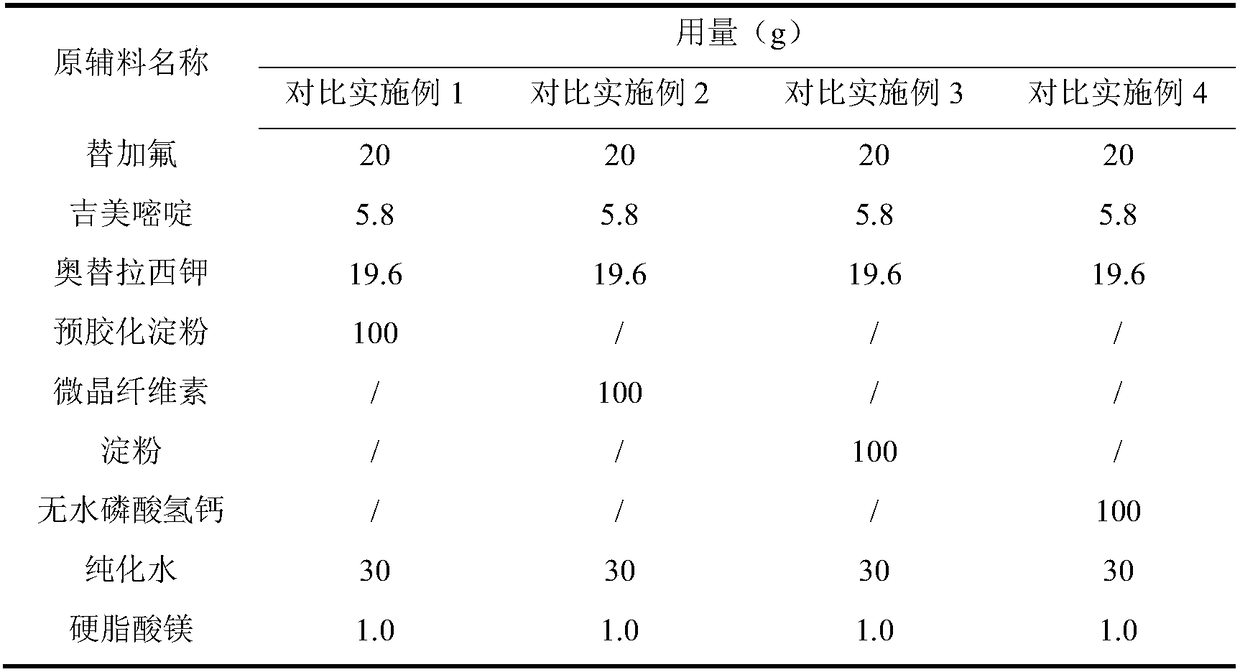
Embodiment 1~5 and comparative Embodiment 1~4
[0047] Embodiments 1 to 5 and comparative examples 1 to 4: determination of dissolution rate
[0048]Adopt dissolution measurement method (Chinese Pharmacopoeia 2015 edition general rule 0931 second method), get the capsule in embodiment 1~5 and comparative example 1~4, take purified aqueous solution as dissolution medium, rotating speed is 50 rpm, temperature is 37±0.5°C, operate according to the law, at 15 minutes, take 10ml of the eluate, filter the eluate with a 0.45μm filter membrane, and use high performance liquid chromatography to determine tegafur, gimeracil and oteracil in S-1 capsules The dissolution rate of potassium is limited to 85% of the labeled amount, and the dissolution results are shown in Table 4.
[0049] Table 4
[0050]
[0051]
[0052] The results of the dissolution test show that the dissolution rate of the active ingredients in Examples 1 to 5 (the filler is lactose or sugar alcohols) is greater than 85% in 15 minutes, and the dissolution rat...
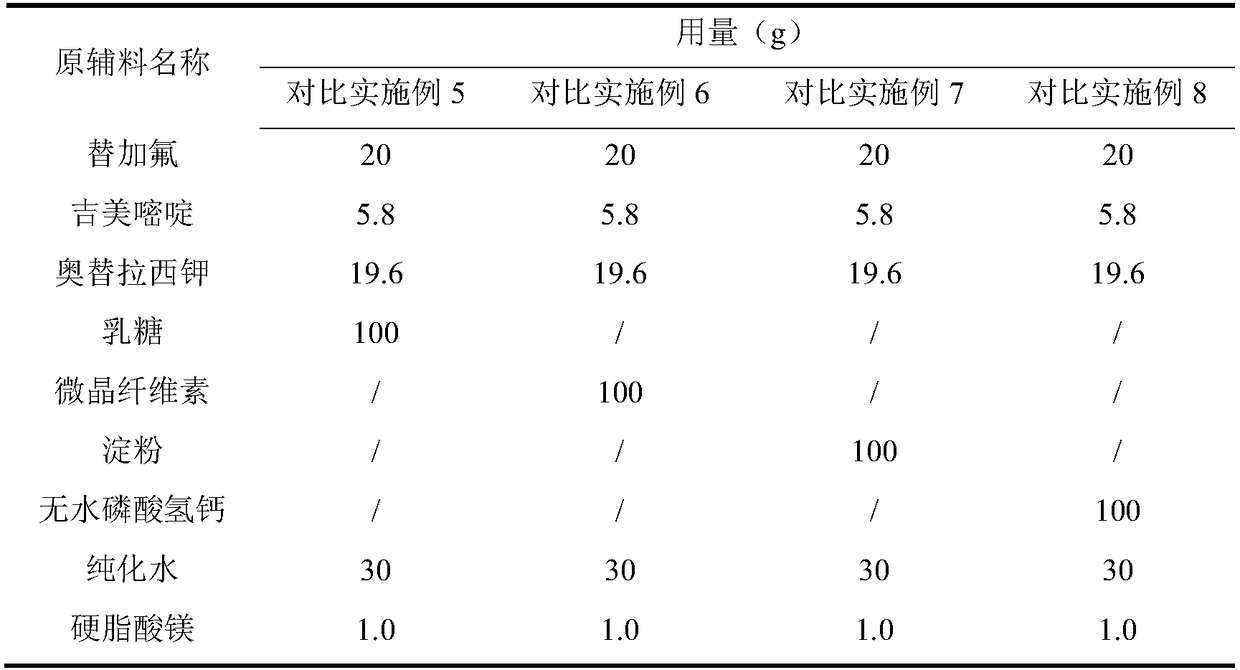
Embodiment 1~5 and comparative Embodiment 5~8
[0053] Embodiment 1~5 and comparative example 5~8: preparation stability investigation
[0054] (1) Influencing factor test: get an appropriate amount of Sigion Capsules in Examples 1 to 5 and Comparative Examples 5 to 8, place them under strong light irradiation (4500lx ± 500lx), high temperature (60°C, high humidity 90%) Placed under the same conditions, samples were taken to check the relevant substances on the 5th and 10th days respectively, and the results are shown in Table 5:
[0055] table 5
[0056]
[0057] (2) Accelerated test: get the Tigio capsules in Examples 1 to 5 and Comparative Examples 5 to 8, place 40°C of temperature under commercially available packaging, and place 6 capsules in a constant temperature and humidity box with a relative humidity of 75%. month, and at the end of the 1st, 2nd, 3rd, and 6th months, the relevant substances were sampled and inspected. The results are shown in Table 6:
[0058] Table 6
[0059]
[0060] Influencing factor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com