New compounding technology for 2,4,6-trimethyl phenylacetyl chloride
A technology of trimethylphenylacetyl and trimethylphenylacetic acid, applied in the new synthesis process field of 2,4,6-trimethylphenylacetyl chloride, which can solve the problem of vacuum degree, high temperature equipment requirements of heat transfer oil, chlorination Eliminates problems such as loss of reagents and large distillation residues, which is not conducive to energy saving and consumption reduction, and achieves the effect of low requirements for production equipment, low price, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
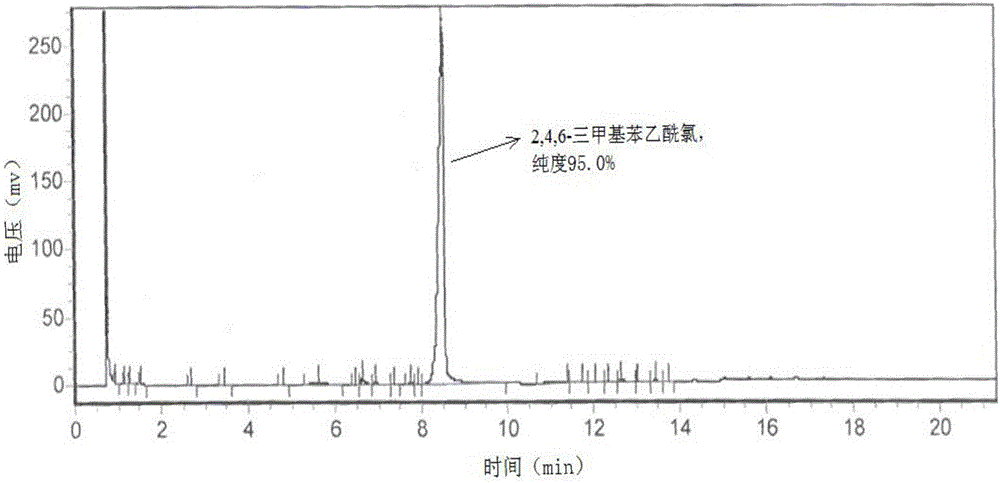
Image
Examples
Embodiment 1
[0060] Step (1), add 166.4g of toluene and 5.0g of N,N dimethylformamide into the reaction kettle, start stirring, add 832g (4.7moL) of 2,4,6-trimethylphenylacetic acid in one batch, mix stir well;
[0061] Step (2), adjust the temperature of the reactor to 15°C, add 565.2g (4.75mol) of thionyl chloride dropwise, and the sulfur dioxide and hydrogen chloride gas produced by the reaction will be processed after passing through the condenser. After the dropwise addition, gradually increase the reaction temperature To 55°C, heat preservation reaction for 5h;
[0062] Step (3), after the reaction is completed, filter the reaction system, and carry out distillation and recovery of toluene at a vacuum degree of 500 Pa and a temperature of 120 ° C. After recovery, the remaining system is filtered under reduced pressure to obtain the product 2,4,6-trimethylbenzene Acetyl chloride.
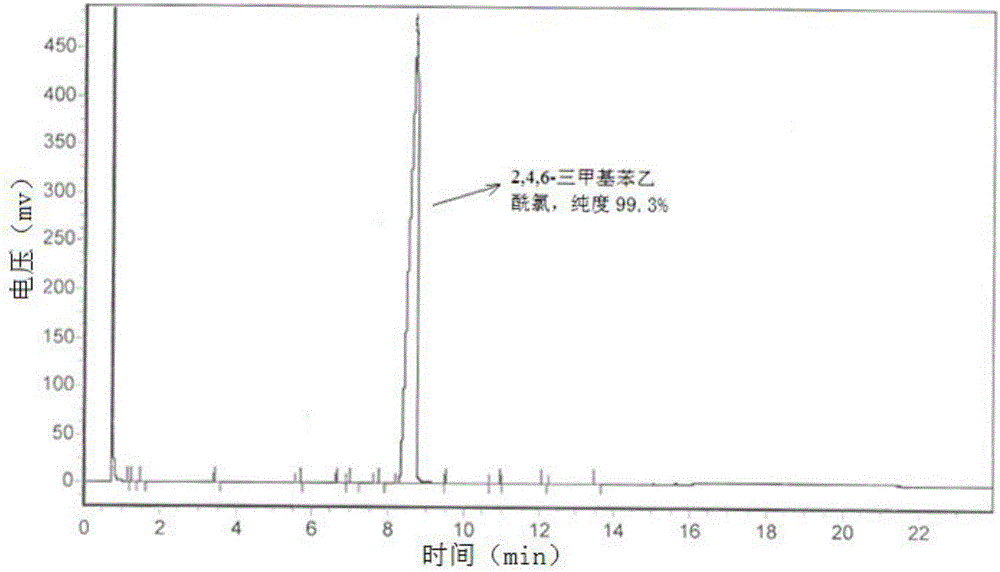
[0063] The product is a yellow liquid. Product in the present embodiment 1 is carried out gas chromat...
Embodiment 2
[0065] Step (1) Add 221.2g of toluene and 4.5g of N,N dimethylformamide into the reaction kettle, start stirring, add 885.0g (5.0moL) of 2,4,6-trimethylphenylacetic acid in one batch, mix stir well;
[0066] Step (2) Adjust the temperature of the reactor to 15°C, add 624.8g (5.25mol) of thionyl chloride dropwise, and then process the sulfur dioxide and hydrogen chloride gas produced by the reaction through the condenser. After the dropwise addition, gradually increase the reaction temperature to 60°C, keep warm for 4 hours;
[0067] Step (3) After the reaction is completed, filter the reaction system, carry out distillation and recovery of toluene at a vacuum degree of 600Pa and a temperature of 115°C, and perform vacuum filtration on the remaining system after recovery to obtain the product 2,4,6-trimethylphenylethyl ether acid chloride.
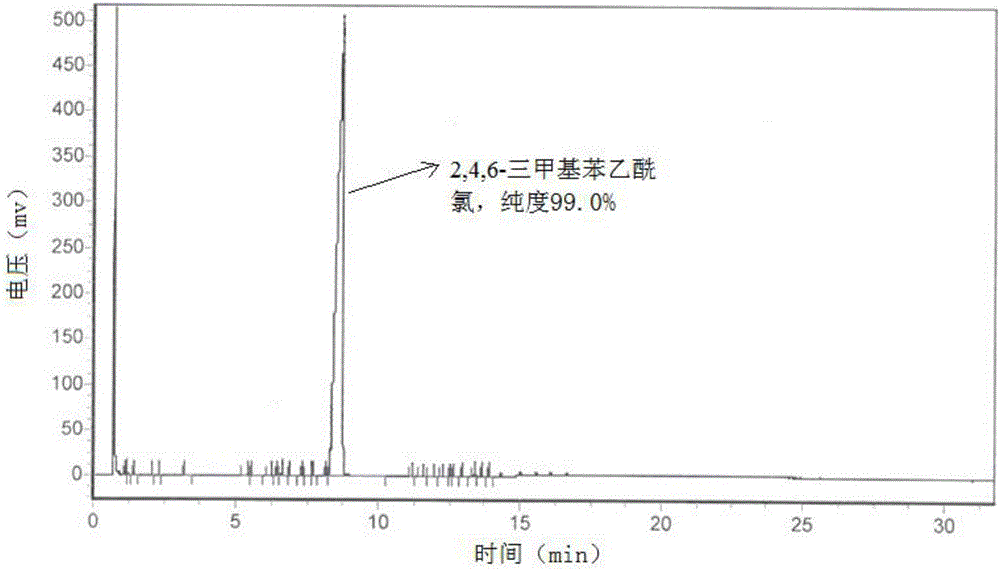
[0068] The product is a yellow liquid. Product in this embodiment 2 is carried out gas chromatography detection, and the result is as f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com