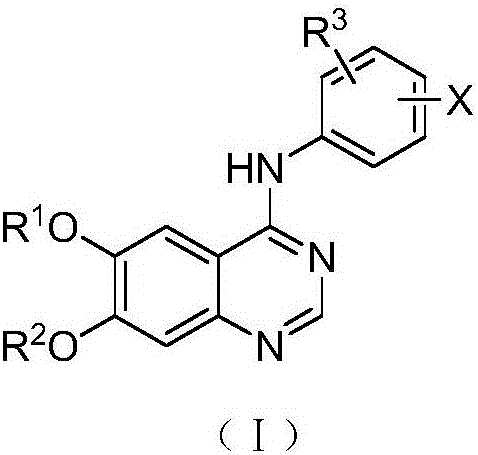
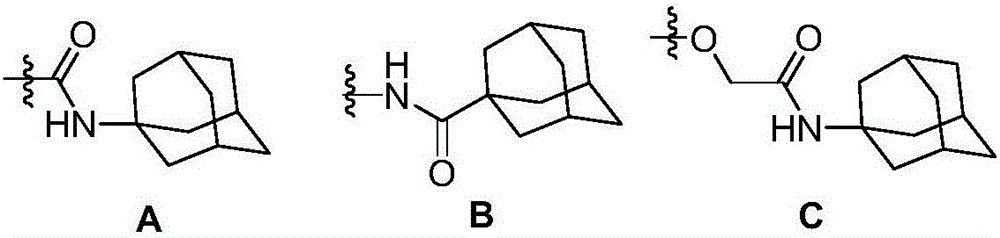
Adamantyl quinazoline compound, composition and application of adamantyl quinazoline compound and composition
An adamantyl quinazoline and adamantyl technology, applied in the field of medicine, can solve the problems of poor drug resistance and the like, and achieve the effect of good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
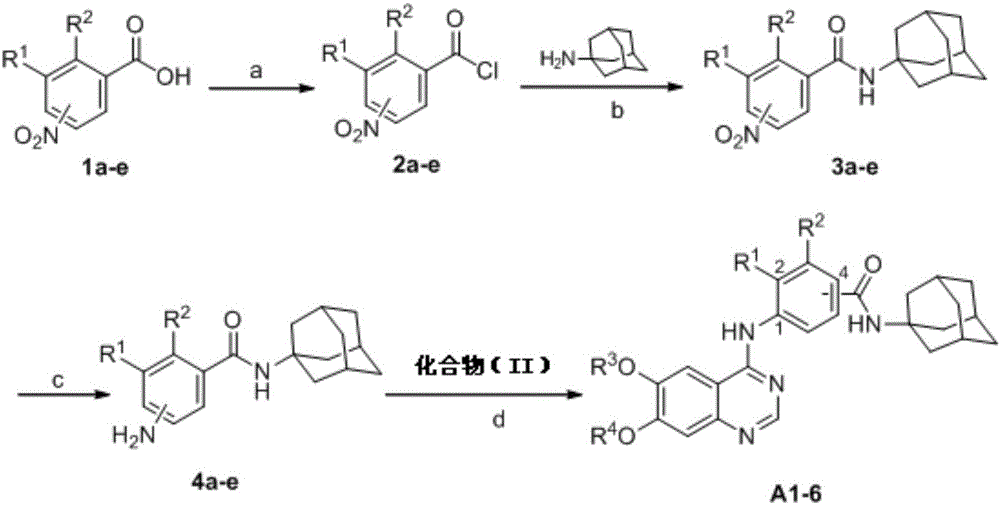
[0061] The reaction path is as follows:
[0062]
[0063] Compound (II):
[0064]
[0065] As above reaction equation, compound A1-6 synthesis method is as follows:
[0066] Step 1 Synthesis of intermediates 2a-e, the reaction conditions are as follows: (a) using raw materials compound 1a-e and thionyl chloride as raw materials, at a constant temperature of 80°C, and reacting for 2 hours;
[0067] Step 2 Synthesis of intermediates 3a-e, the reaction conditions are: (b) use intermediates 2a-e and 1-aminoadamantane as raw materials, and reflux in acetone solution of sodium bicarbonate for 2 hours;
[0068] Step 3 Synthesis of intermediates 4a-e, the reaction conditions are: (c) use intermediates 3a-e and ammonium chloride as raw materials, iron powder as a catalyst, and react in methanol aqueous solution at a constant temperature of 80°C for 6 hours;
[0069] Step 3 Synthesis of compound A1-6, the reaction conditions are: (d) Using intermediate 4a-e and raw material comp...
Embodiment 2
[0086] The reaction path is as follows:
[0087]
[0088] Compound (II):
[0089]
[0090] As above reaction equation, compound B1-6 synthesis method is as follows:
[0091] Step 1 Synthesis of intermediates 6a-e, the reaction conditions are: (a) using raw material compounds 5a-e and 1-adamantanyl chloride as raw materials, refluxing in acetone solution of sodium bicarbonate for 2 hours;
[0092] Step 2 Synthesis of intermediates 7a-e, the reaction conditions are: (b) use intermediates 6a-e and ammonium chloride as raw materials, iron powder as a catalyst, and react in methanol aqueous solution at a constant temperature of 80°C for 6 hours;
[0093] The reaction conditions for the synthesis of the product B1-6 in Step 3 are as follows: (c) Use the intermediate 7a-e and the raw material compound (II) as raw materials, and reflux in a methanol solvent for 2 hours.
[0094] The selection of the compound B1-6 substituent is as follows:
[0095]
[0096]
[0097] The...
Embodiment 3
[0111] The reaction path is as follows:
[0112]
[0113] Compound (II):
[0114]
[0115] As above reaction equation, compound C1-12 synthesis method is as follows:
[0116] Step 1 Synthesis of intermediate 9a-e, the reaction conditions are: (a) use raw material compound 8a-e, ethyl bromoacetate, and potassium carbonate as raw materials, reflux in acetone solvent for 2h; and then (b) add 1mol / L hydrogen Sodium oxide solution, stirred at room temperature for 2 hours;
[0117] Step 2 Synthesis of intermediates 10a-e, the reaction conditions are: (c) use intermediates 9a-e and oxalyl chloride as raw materials, and reflux for 2 hours; then (d) add 1-adamantanamine and sodium carbonate, and reflux in acetone 2h;
[0118] Step 3 Synthesis of intermediates 11a-e, the reaction conditions are: (e) Use intermediates 10a-e and ammonium chloride as raw materials, iron powder as a catalyst, and react in methanol aqueous solution at a constant temperature of 80°C for 6 hours;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com