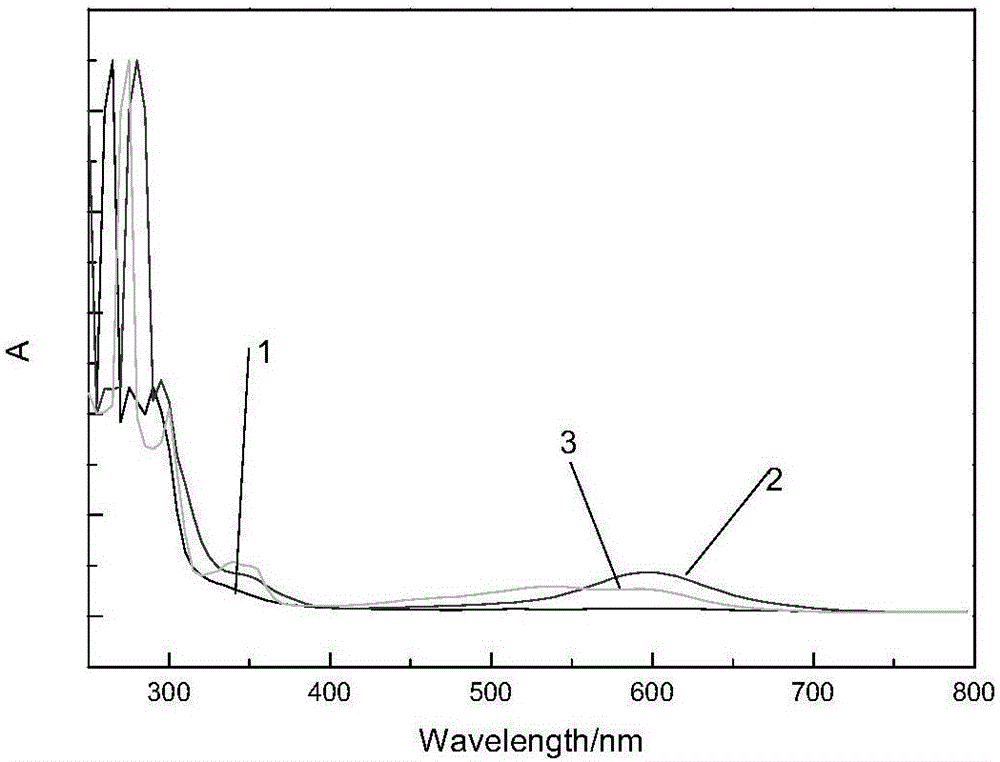
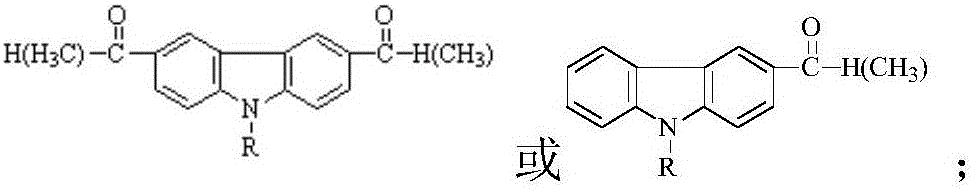
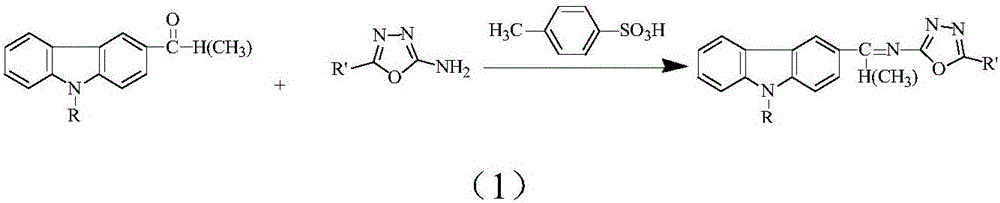
Schiff base containing carbazolyl group and oxadiazole group and preparing method thereof
An oxadiazolyl and carbazole-based technology, which is applied in the field of chemical synthesis, can solve the problems of large solvent usage, long reaction time, and low yield, and achieve the effects of short reaction time, fast reaction speed, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1) Add 0.005mol 3,6-dimethylacyl-9-methylcarbazole, 0.011mol 2-amino-5-phenyl-1,3,4-oxadiazole and 0.011mol p-toluenesulfonic acid, that is, 3,6-dimethylacyl-9-methylcarbazole: 2-amino-5-phenyl-1,3,4-oxadiazole: p-toluenesulfonic acid is 1:2.2: 2.2; Grinding at room temperature for 15 minutes, TLC monitoring at this time showed that the raw material sites of 3,6-diformyl-9-methylcarbazole and 2-amino-5-phenyl-1,3,4-oxadiazole disappeared, Indicates that the raw materials are completely reacted, and then left to stand to remove water for 30 minutes to obtain a mixture; wherein the developer is a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3;
[0039] 2) After the mixture was washed with water and suction filtered, 3,6-diformyl-9-methylcarbazole 2-amino-5-phenyl-1,3,4-oxadiazole Schiff base was obtained. m.p.: 252.7-254.9°C, yield 88.0%.
[0040] IR (KBr tablet): 3067 (Ar-H); 2974 (saturated C-H); 1642 (C=N), 1592, 1479 (benzene ring skel...
Embodiment 2
[0045] 1) Add 0.005mol 3-formyl-9-methylcarbazole, 0.006mol 2-amino-5-p-chlorophenyl-1,3,4-oxadiazole and 0.006mol p-toluene to a dry mortar Sulphonic acid was ground at room temperature for 16 min. At this time, TLC monitoring showed that the raw material sites of 3-formyl-9-methylcarbazole and 2-amino-5-p-chlorophenyl-1,3,4-oxadiazole disappeared, Indicates that the raw materials are completely reacted, and then left to stand for 30 minutes to obtain a mixture; wherein the developer is a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3;
[0046] 2) After the mixture was washed with water and suction filtered, 3-formyl-9-methylcarbazole 2-amino-5-p-chlorophenyl-1,3,4-oxadiazole Schiff base was obtained. m.p.: 171.6-175.5°C, yield 83.2%.
[0047] IR (KBr tablet): 3092 (Ar-H); 2988 (saturated C-H); 1623 (C=N); 1598, 1488 (benzene ring skeleton vibration);
[0048] 1369(-CH 3 ); 1244(C-N); 1079(C-O-C), 688(C-Cl)
[0049] 1 H NMR (CDCl 3 -d 6 ,...
Embodiment 3
[0053] 1) Add 0.005mol 3,6-diacetyl-9-ethylcarbazole, 0.011mol 2-amino-5-p-chlorophenyl-1,3,4-oxadiazole and 0.011mol to a dry mortar mol of p-toluenesulfonic acid, ground for 15min at room temperature, at this time TLC monitoring showed that 3,6-diacetyl-9-ethylcarbazole and 2-amino-5-p-chlorophenyl-1,3,4-oxadi The raw material point of the azole disappears, indicating that the raw material is completely reacted, and then left to stand for 30 minutes to obtain a mixture; wherein the developing agent is a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of 1:3;
[0054] 2) After the mixture was washed with water and suction filtered, 3,6-diacetyl-9-ethylcarbazole 2-amino-5-p-chlorophenyl-1,3,4-oxadiazole Schiff base was obtained. m.p.: 252.3-257.5°C, yield 87.6%.
[0055] IR (KBr tablet): 3089 (Ar-H); 2990, 2872 (saturated C-H); 1598 (C=N); 1588 (benzene ring skeleton); 1476
[0056] (-CH 2 -);1369(-CH 3 ); 1236(C-N); 1089(C-O-C); 692(C-Cl)
[0057] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com