Crystalline compound of drug ceftriaxone sodium for treating surgical operation infections
The technology of a crystal compound, ceftriaxone sodium, is applied in the field of medicine and achieves the effects of good fluidity, high yield and strong repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 ceftriaxone sodium crystal compound
[0046] (1) Dissolve the crude product of ceftriaxone sodium into a mixed solution of water and carbon tetrachloride whose volume is 8 times the weight of ceftriaxone sodium at 35°C, and the volume ratio of the water and carbon tetrachloride is 4:2.5 ;
[0047] (2) add activated carbon whose weight is 0.2 times of the weight of ceftriaxone sodium for decolorization, filter to obtain ceftriaxone sodium solution;
[0048] (3) Raise the temperature of the ceftriaxone sodium solution to 40°C, and add propyl ether dropwise to the ceftriaxone sodium solution under the condition of stirring. Complete, the stirring rate is 30rmp;
[0049] (4) After the dropwise addition, cool down to -10°C at a rate of 15°C / hour, continue to stir at a stirring rate of 15rmp for 2h, let stand for 3h to precipitate crystals, filter, wash, and vacuum-dry to obtain ceftriaxone sodium crystals.
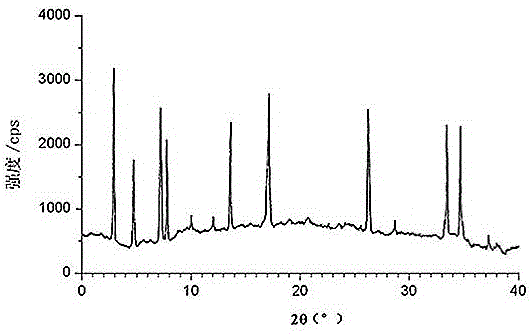
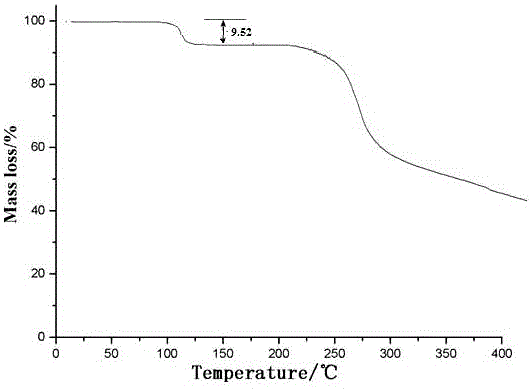
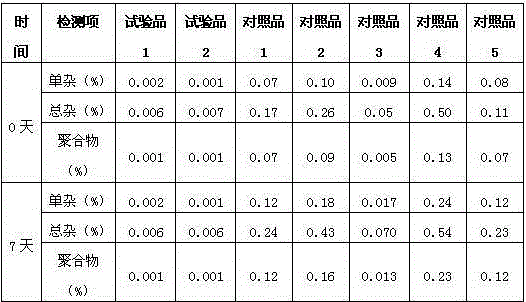
[0050] determined by powder X-ra...
Embodiment 2
[0053] The preparation of embodiment 2 ceftriaxone sodium crystalline compound
[0054] (1) Dissolve the crude product of ceftriaxone sodium into a mixed solution of water and carbon tetrachloride whose volume is 9 times the weight of ceftriaxone sodium at 35°C, and the volume ratio of the water and carbon tetrachloride is 4:2.5 ;
[0055] (2) adding activated carbon whose weight is 0.3 times of the weight of ceftriaxone sodium for decolorization, and filtering to obtain ceftriaxone sodium solution;
[0056] (3) Raise the temperature of the ceftriaxone sodium solution to 40°C, and add propyl ether dropwise to the ceftriaxone sodium solution under the condition of stirring. Complete, the stirring rate is 30rmp;
[0057] (4) After the dropwise addition, cool down to -10°C at a rate of 15°C / hour, continue to stir at a stirring rate of 15rmp for 2h, let stand for 3h to precipitate crystals, filter, wash, and vacuum-dry to obtain ceftriaxone sodium crystals.
[0058] Measure wit...
Embodiment 3
[0059] The preparation of embodiment 3 ceftriaxone sodium crystalline compound
[0060] (1) Dissolve the crude product of ceftriaxone sodium into a mixed solution of water and carbon tetrachloride whose volume is 10 times the weight of ceftriaxone sodium at 35°C, and the volume ratio of the water and carbon tetrachloride is 4:2.5 ;
[0061] (2) add activated carbon whose weight is 0.4 times of the weight of ceftriaxone sodium for decolorization, and filter to obtain ceftriaxone sodium solution;
[0062] (3) Raise the temperature of the ceftriaxone sodium solution to 40°C, and add propyl ether dropwise to the ceftriaxone sodium solution under the condition of stirring. Complete, the stirring rate is 30rmp;
[0063] (4) After the dropwise addition, cool down to -10°C at a rate of 15°C / hour, continue to stir at a stirring rate of 15rmp for 2h, let stand for 3h to precipitate crystals, filter, wash, and vacuum-dry to obtain ceftriaxone sodium crystals.
[0064] Measure with pow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com