Recombination microorganism for producing beta-carotene, construction method and application
A technology of carotene and recombinant bacteria, applied in the biological field, can solve the problem of reducing expression intensity and producing β-carotene by recombinant Escherichia coli
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] The preparation method of salt-free LB is as follows:
[0068] 50% sucrose solution: Weigh 500g of sucrose, dissolve it in a small amount of ultrapure water, and dilute to 1L, sterilize at 115°C for 20 minutes.
[0069] 10% salt-free sucrose LB medium: Weigh 5g of yeast extract, 10g of peptone in 800ml of water, sterilize at 115°C for 20min. Add 200ml of 50% sucrose solution after sterilization.
[0070] 6% salt-free sucrose LB medium: Weigh 5g yeast extract, 10g peptone, 15g agar powder, dissolve in 880ml water, sterilize at 115°C for 20min. Add 120ml of 50% sucrose solution after sterilization.
[0071] The concentrations of chloramphenicol, ampicillin, and kanamycin in the examples were 34 μg / L, 50 μg / L, and 50 μg / L, respectively.
[0072] Primers used in the present invention in table 1
[0073]
[0074]
[0075]
[0076] Table 2 is the bacterial strain constructed in the present invention
[0077]
[0078]
[0079]
[0080]
Embodiment 1
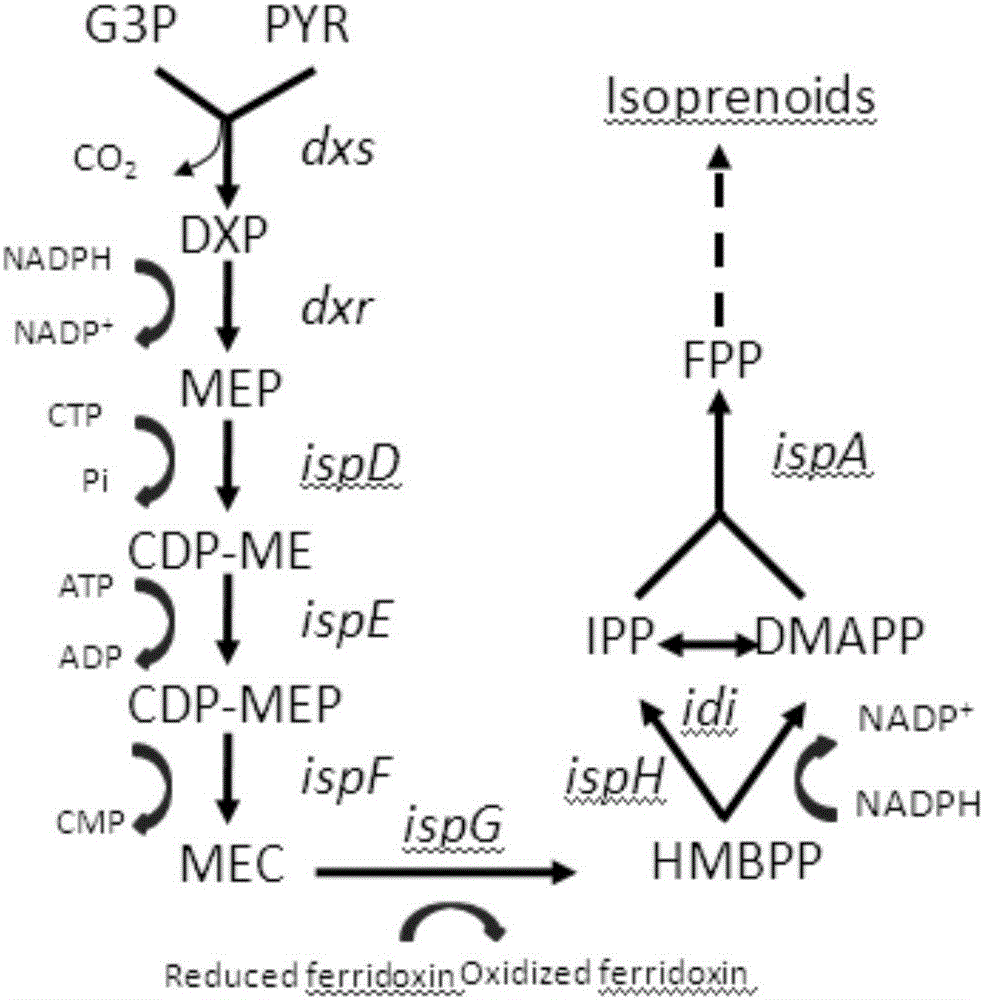
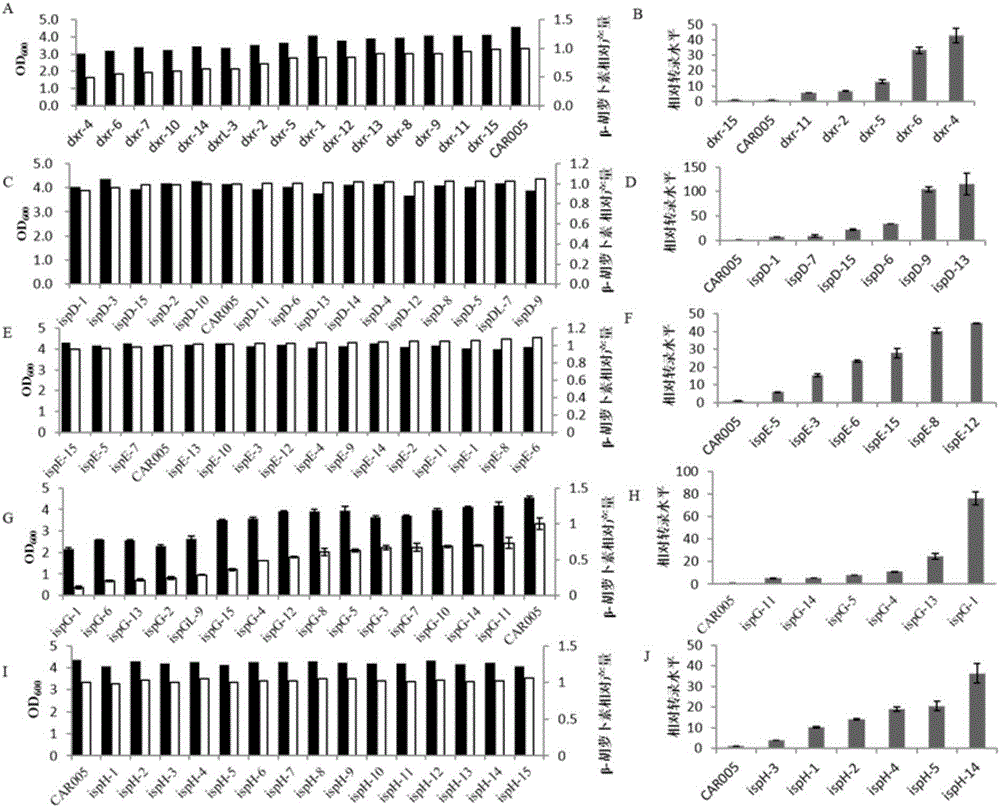
[0081] Embodiment 1, explore the relationship between MEP pathway dxr, ispD, ispE, ispG and ispH gene expression intensity and β-carotene output
[0082] In order to study the MEP pathway in CAR005 (Zhao J, Li Q, Sun T, et al. Engineering central metabolic modules of Escherichia coli for improving beta-carotene production. Metabolicengineering 2013; 17:42-50. Both CAR001 and CAR005 are from this article) The relationship between the expression intensity of each gene and the production of β-carotene, the mRS library was established to regulate dxr, ispD, ispE, ispG and ispH genes, 15 strains were randomly selected, and the production of β-carotene was determined. According to the production of β-carotene, Then select several representative strains and use the real-time quantitative PCR method to measure the gene expression level, and study the relationship between the expression level and the yield of β-carotene.
[0083] 1. Construction of mRS library to regulate dxr, ispD, is...
Embodiment 2
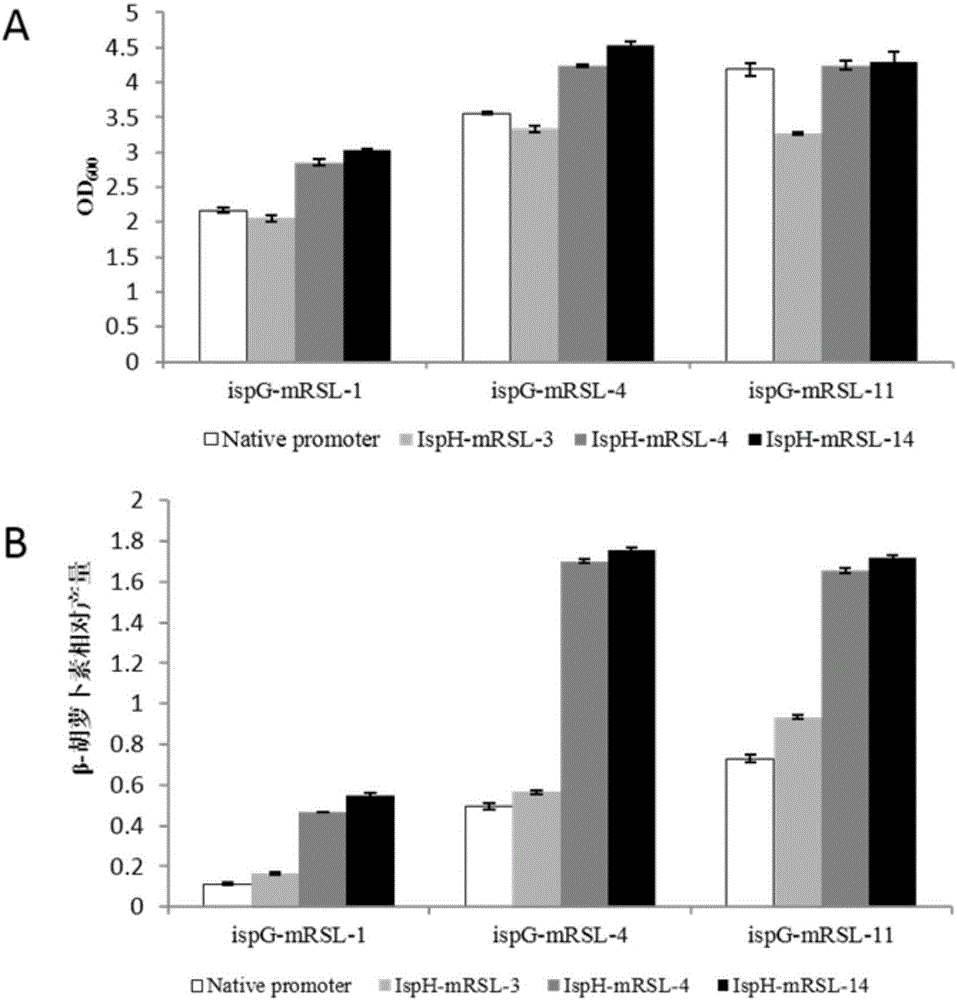
[0125] Embodiment 2, the recombinant bacterium obtained by combination regulation of ispG and ispH
[0126] The ispG artificial regulatory elements of ispG-1, ispG-4, and ispG-11 in the ispG library regulation, and the ispH artificial regulatory elements of ispH-3, ispH-4, and ispH-14 in the ispH library were combined and regulated in CAR005 .
[0127] 1. Construction of recombinant Escherichia coli G1H3, G1H4, G1H14, G4H3, G4H4, G4H14, G11H3, G11H4 and G11H14
[0128]Recombinant Escherichia coli G1H3 is the mRSL regulatory element mRSL-1::ispG (SEQ ID NO: 21) that pre-inserts the ispG gene in the genome of the starting bacteria CAR005 to start the expression of the ispG gene, and inserts the front of the ispH gene in the genome of the starting bacteria CAR005 to start the ispH gene Expressed mRSL-3::ispH (SEQ ID NO: 29);
[0129] Recombinant Escherichia coli G1H4 is the mRSL regulatory element mRSL-1::ispG (SEQ ID NO: 21) that pre-inserts the ispG gene in the genome of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com