Myoglobin monoclonal abzyme marking compound and preparation method thereof and detection test kit
A monoclonal antibody and detection kit technology, applied in the field of in vitro diagnostic medical testing, can solve the problems of low sensitivity, difficulty in controlling batch-to-batch differences, unfavorable promotion of myoglobin detection, etc., achieve high detection sensitivity, increase reaction signal, and result good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
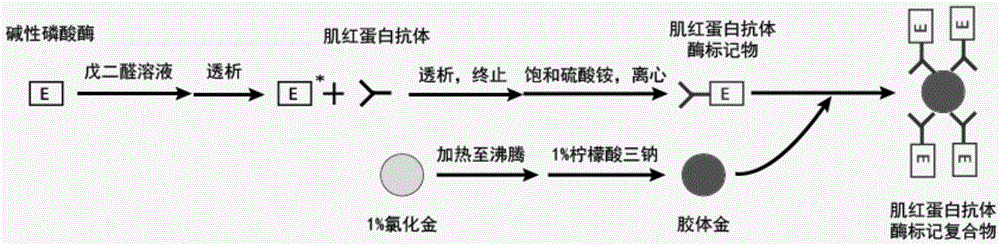
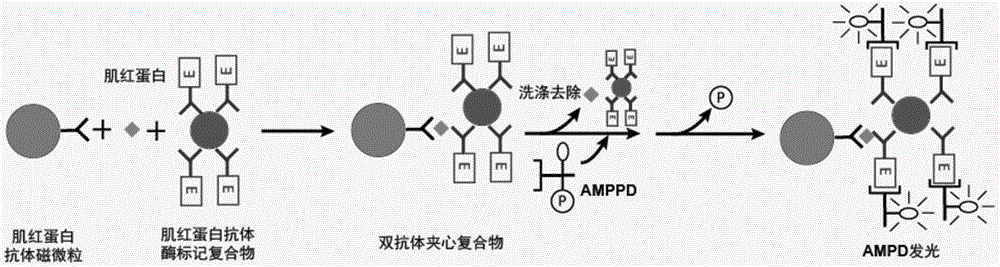
[0065] Correspondingly, the present invention also provides a method for preparing the myoglobin monoclonal antibody enzyme-labeled complex, the method comprising the following steps:
[0066] 1) cross-linking myoglobin monoclonal antibody with enzyme to prepare antibody enzyme marker;
[0067] 2) adding the abzyme-labeled substance obtained in step 1) into the colloidal gold solution, thereby obtaining the myoglobin monoclonal abzyme-labeled complex.
[0068] In a specific embodiment, the method includes:
[0069] a) the myoglobin monoclonal antibody and the enzyme are cross-linked by the glutaraldehyde method to prepare antibody enzyme markers;
[0070] b) adding trisodium citrate in gold chloride solution to prepare colloidal gold solution;
[0071] c) adding the abzyme marker reacted in step a) to the colloidal gold solution, mixing evenly, and adding the blocking solution;
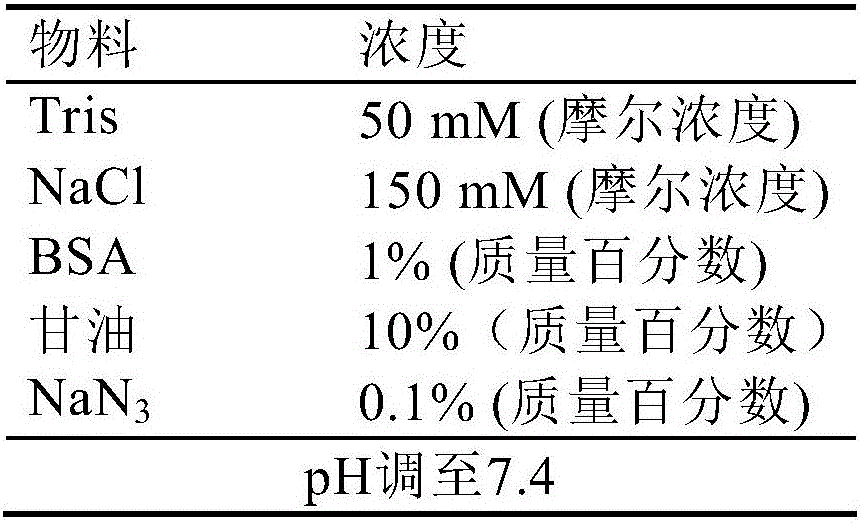
[0072] d) Preserving the antibody marker treated in step c) in the magnetic particle preservati...
Embodiment 1
[0143] Example 1. Preparation of myoglobin monoclonal antibody enzyme-labeled complex
[0144] 1) Dissolving alkaline phosphatase in a final concentration of 5% glutaraldehyde solution, so that the concentration of alkaline phosphatase is 10 mg / mL;
[0145] 2) Stand overnight at room temperature;
[0146] 3) Dialysis with normal saline (volume ratio greater than 1:200) for at least 4 hours;
[0147] 4) Take out the dialyzed alkaline phosphatase, add myoglobin monoclonal antibody according to the mass ratio of 1:1, and mix well;
[0148] 5) Dialyze in 50mM pH 9.6 sodium carbonate-sodium bicarbonate buffer overnight;
[0149] 6) Take out the dialysate, add 1M glycine solution according to the volume ratio of 1:1000, mix well, and stop the reaction for 2 hours;
[0150] 7) Add an equal volume of saturated ammonium sulfate and store at 2-8°C for 2 hours;
[0151] 8) Centrifuge at 4000rpm to remove the supernatant, and dissolve the precipitate in 50mM pH 7.40 phosphate buffer;
...
Embodiment 2
[0157] Example 2. Sensitivity detection of myoglobin monoclonal antibody enzyme-labeled complex
[0158] Using the myoglobin monoclonal antibody enzyme-labeled complex obtained in Example 1, the sensitivity detection was carried out as described in the materials and methods, and the results are shown in the following table:
[0159]
[0160] Note:
[0161] (1) Traditional myoglobin monoclonal antibody enzyme markers;
[0162] (2) Using colloidal gold-coated myoglobin monoclonal antibody enzyme-labeled complex.
[0163] As can be seen from the above table, using the colloidal gold-coated myoglobin monoclonal antibody enzyme-labeled complex of the present invention, its sensitivity (S / N value) has increased by 1.92 times than traditional myoglobin monoclonal antibody enzyme-labeled substances . In addition, the functional sensitivity of its detection is 0.5 ng / mL, which is significantly lower than that of traditional myoglobin monoclonal antibody enzyme markers. To sum up...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com