A kind of porcine circovirus type ii genetic engineering subunit vaccine and its application
A porcine circovirus, virus-like technology, applied in the direction of genetic engineering, application, viral peptides, etc., can solve the problems of cost reduction and popularization and application, high process cost, unfavorable vaccines, etc., and achieve low cost, broad application prospects, and antigen purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Sequence Synthesis
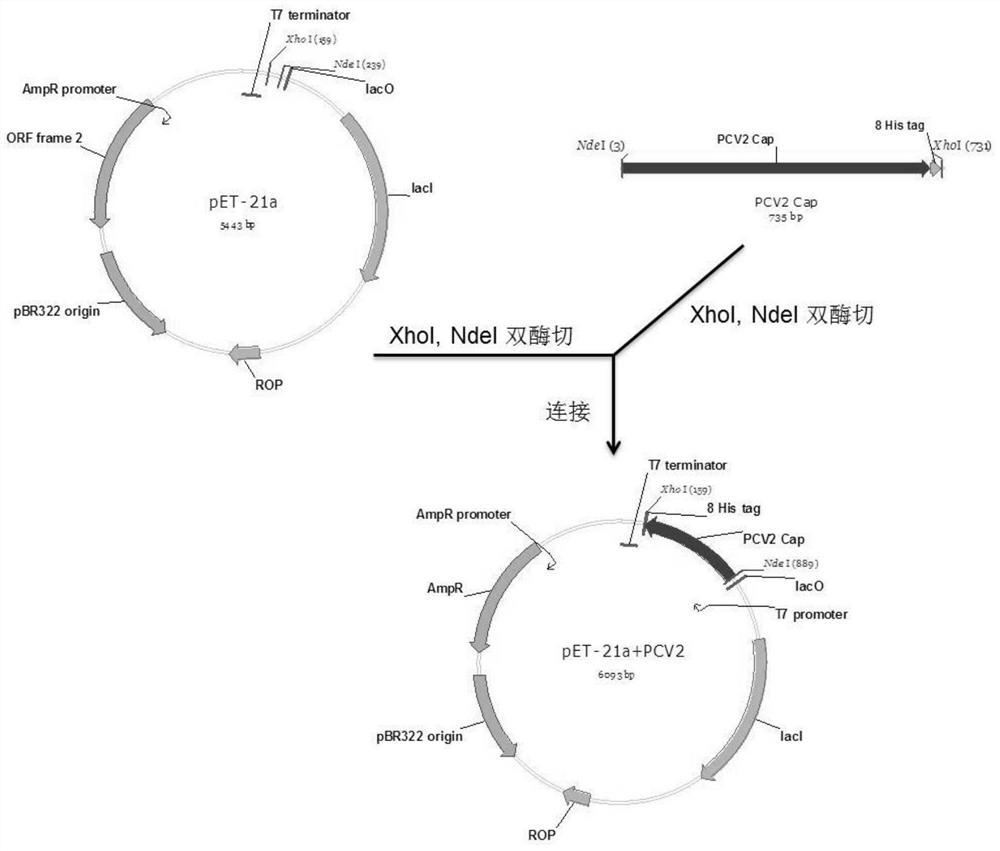
[0024] Through sequence comparison, the nucleotide sequence was changed under the premise of ensuring the same encoded amino acid, the rare codons in the sequence were replaced with E. coli preferred codons, and a 6His or 8His tag and a stop codon were added to the 3' end of the PCV2ORF2 gene sequence The 5' end of the sequence is the Nde I restriction endonuclease recognition site, and the 3' end is the Xho I restriction endonuclease recognition site. All the above sequences are artificially synthesized sequences. The synthetic sequence was digested with Nde I and Xho I and connected to the pET-21a(+) vector (see figure 1 ).
Embodiment 2
[0025] Example 2 Construction of expression vector pET-21a-PCV2-ORF2
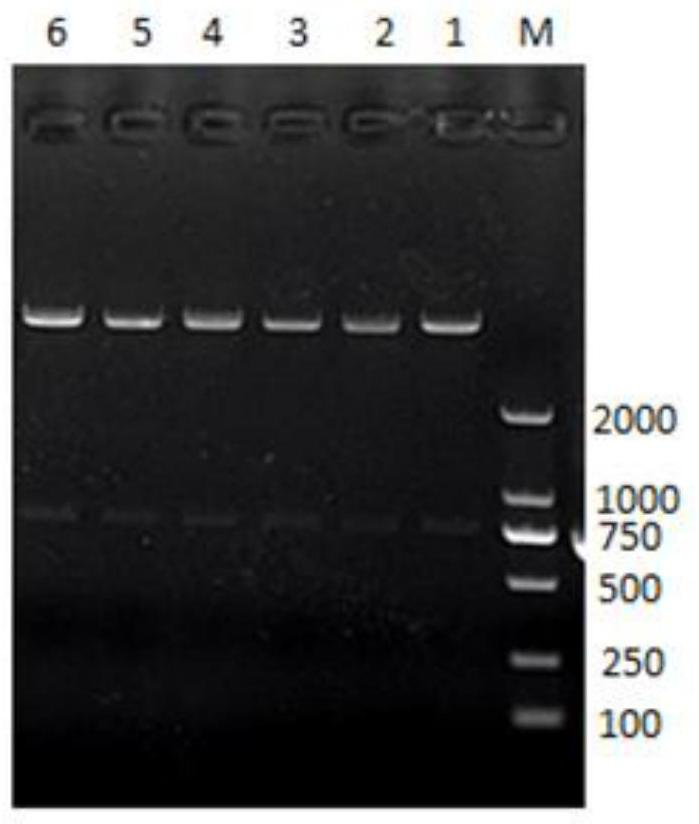
[0026] Digest the recombinant plasmid with restriction endonucleases Nde I and Xho I, separate the digested products by agarose gel electrophoresis, and recover small fragments by cutting the gel (see figure 2 ), and then subcloned into the linearized vector pET-21a(+), the vector linearization process uses the same restriction endonuclease, the connection solution is transformed into Escherichia coli DH5α, and the positive clones are screened and verified by sequencing. After the frames are correct, the recombinant vectors are named pET-21a-PCV2-ORF2-6 His and pET-21a-PCV2-ORF2-8His respectively, and transformed into BL21(DE3) competent cells for protein expression.
Embodiment 3
[0027] Induced expression of embodiment 3 recombinant protein
[0028] (1) Pick the recombinant expression strains pET-21a-PCV2-ORF2-6His and pET-21a-PCV2-ORF2-8His and inoculate scattered single colonies in LB liquid medium containing ampicillin, and shake overnight at 37°C .
[0029] (2) Transfer the positive BL21 bacterial solution to the LB liquid medium containing Amp at a ratio of 1:100, culture it with shaking at 37°C until the logarithmic growth phase (OD600 reaches 0.6-0.8), take 100μl sample in In a sterile Eppendorf tube as a pre-induction control;
[0030] (3) Add IPTG with a final concentration of 1.0mmol / L to the above bacterial solution to induce expression of the recombinant protein, and take samples at 3, 4, 5, 6, 7, and 8 hours after adding IPTG;
[0031] (4) Centrifuge at 12,000 rpm at 4°C for 5 minutes to collect the bacteria induced to express, resuspend in PBS, and wash twice;
[0032] (5) Discard the supernatant completely, and resuspend the bacterial...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com