A kind of ophthalmic pharmaceutical composition
A composition and drug technology, applied in the field of ophthalmic pharmaceutical compositions, can solve the problems of inability to effectively control heavy metal content, unclear action mechanism, unsatisfactory and the like, meet clinical treatment needs, relieve eye dryness, protect The effect of corneal epithelial histiocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of eye drops containing polyvinyl alcohol with a mass concentration of 0.15%, a pH value of 6.5 to 7.0, and an osmolarity ratio of 1.0, the steps are as follows:
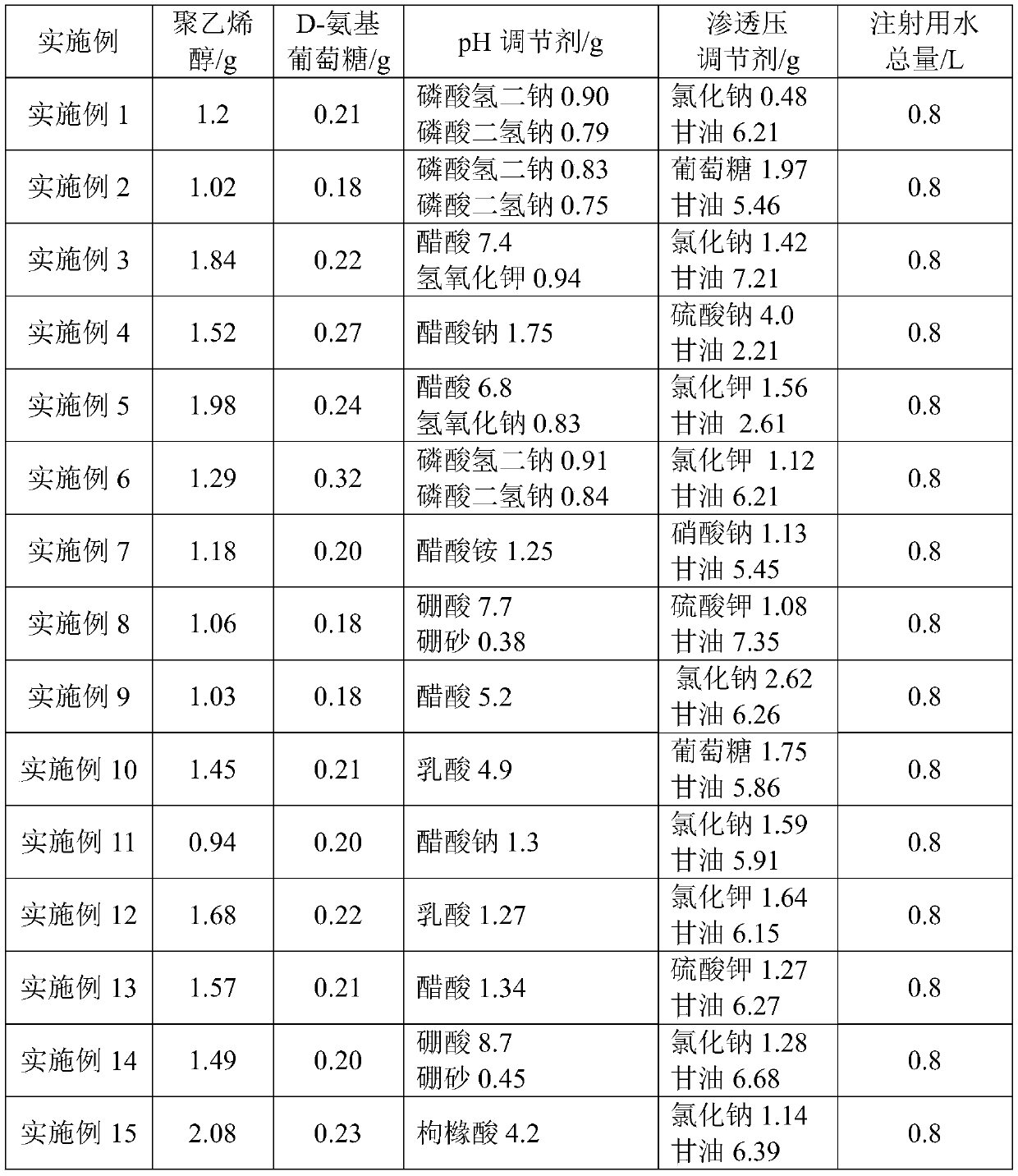
[0041] (1) Accurately weigh 1.2 g of polyvinyl alcohol as described in Table 1, add it to a 100L batching tank, raise the temperature to 130°C, and dissolve it in water for injection at a stirring rate of 90r / min to obtain solution A;
[0042] (2) Accurately weigh 0.25g D-glucosamine, 10.21g glycerin, 0.48g sodium chloride, 0.90g disodium hydrogen phosphate and 0.79g sodium dihydrogen phosphate in sequence, and add them to a 50L batching tank with a stirring rate of 90r / min , after being dissolved in water for injection, solution B was obtained, which was transferred to a 100L batching tank;
[0043] (3) Add water for injection to 800mL of the combined solution C, stir evenly, pass through a 5μm titanium rod filter, 0.45μm microporous membrane, 0.22μm microporous membrane, and aseptically fill to ...
Embodiment 16
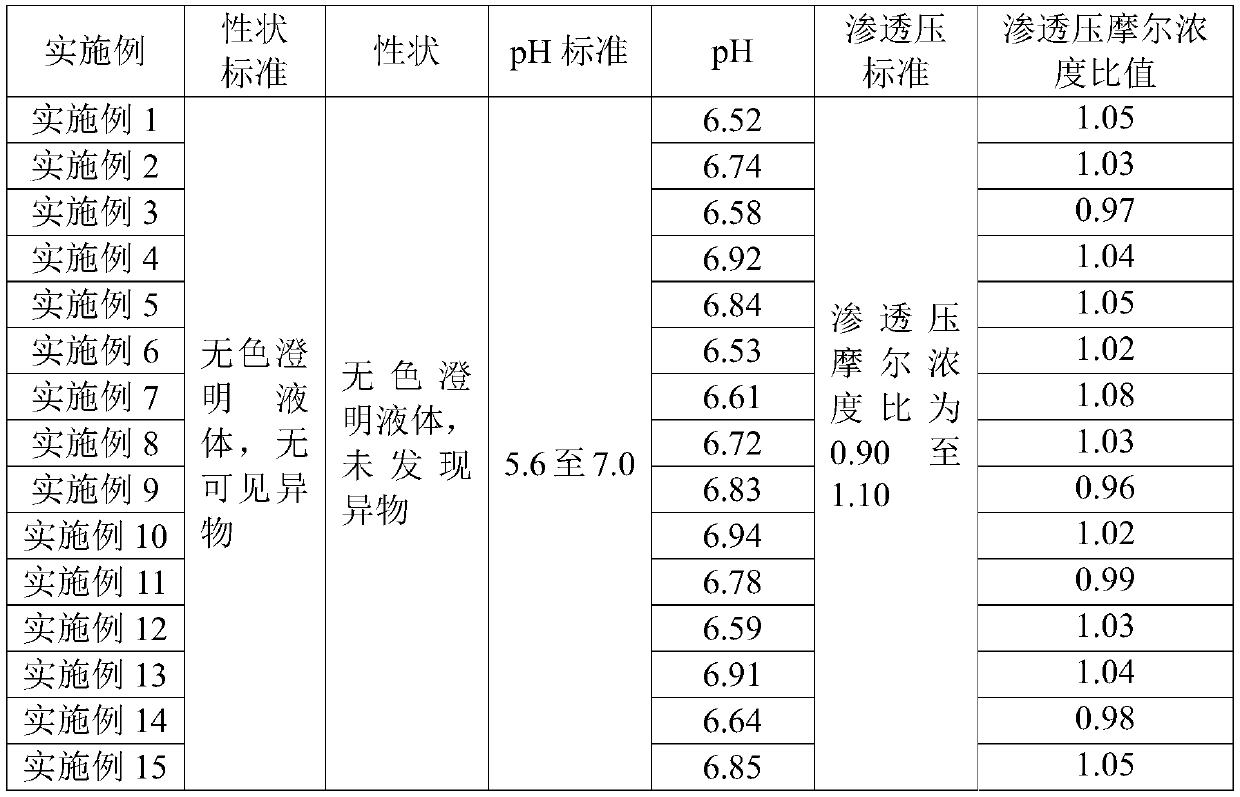
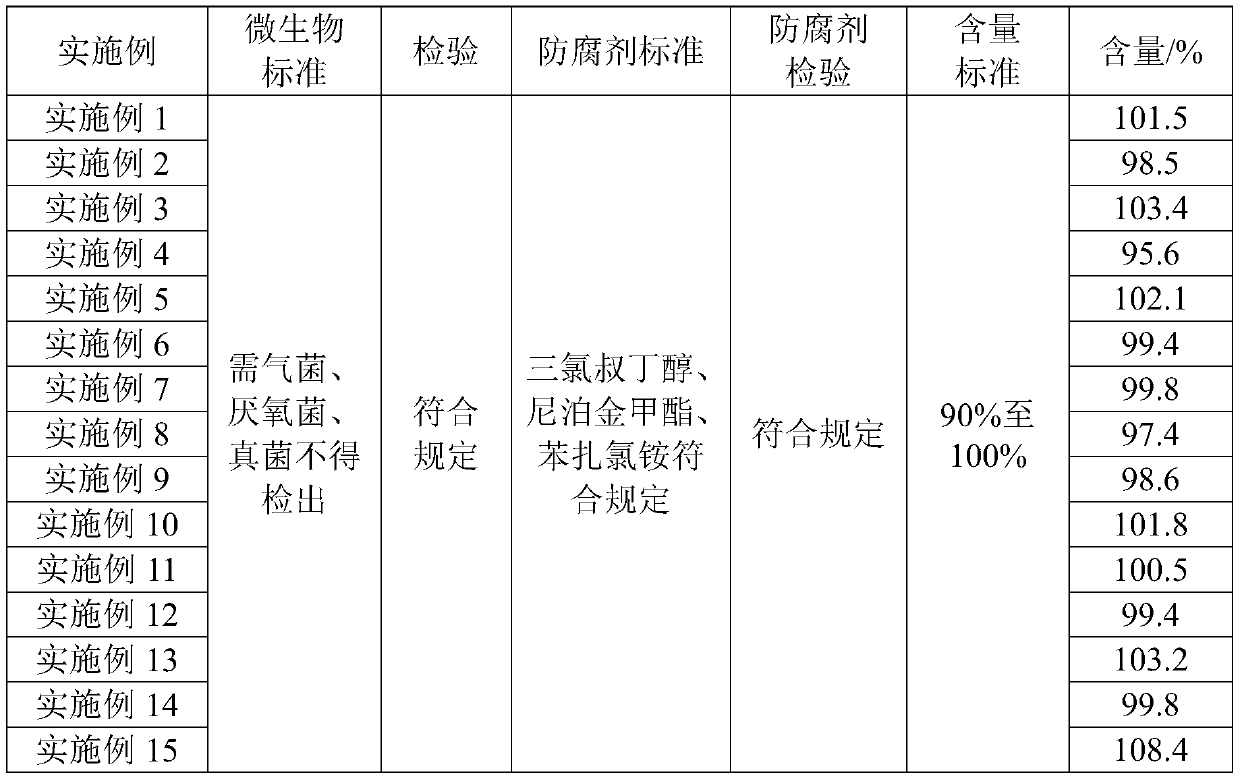
[0056] The composition eye liquid preparations prepared in Examples 1 to 15 were tested according to the quality requirements for ophthalmic preparations stipulated in Part Four of the Pharmacopoeia of the People's Republic of China (2015 Edition), and the test results are shown in Table 2 and Table 3.
[0057] Table 2 Embodiment 1-15 part inspection results
[0058]
[0059] Table 3 Embodiment 1-15 part inspection results
[0060]
[0061] It can be seen from Table 2 and Table 3 that the prepared Examples 1 to 15 all meet the quality requirements for ophthalmic preparations stipulated in Part Four of the Pharmacopoeia of the People's Republic of China (2015 Edition).
Embodiment 17
[0063] The composition ophthalmic solution preparation that embodiment 1 to 15 is prepared is carried out clinical test, randomly selects the symptom caused by tear reduction, tiredness, dry eye, Sjogren's syndrome, keratoconjunctivitis sicca, Stein-John II corneal surgery including eyelid insufficiency or sensory nerve palsy, concomitant strain conjunctivitis, viral conjunctivitis, laser keratectomy, cataract surgery and 900 patients with eye dryness symptoms caused by wearing contact lenses etc. were randomly divided into 18 groups, and groups 1-15 corresponded to using the composition eye liquid preparations prepared in 1-15 in the examples respectively, and the control group 1 Use the composition eye liquid preparation prepared in Comparative Example 1, the matched group 2 uses the composition eye liquid preparation prepared in Comparative Example 2, the matched group 3 uses the composition eye liquid preparation prepared in Comparative Example 3, and the matched group 3 us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com