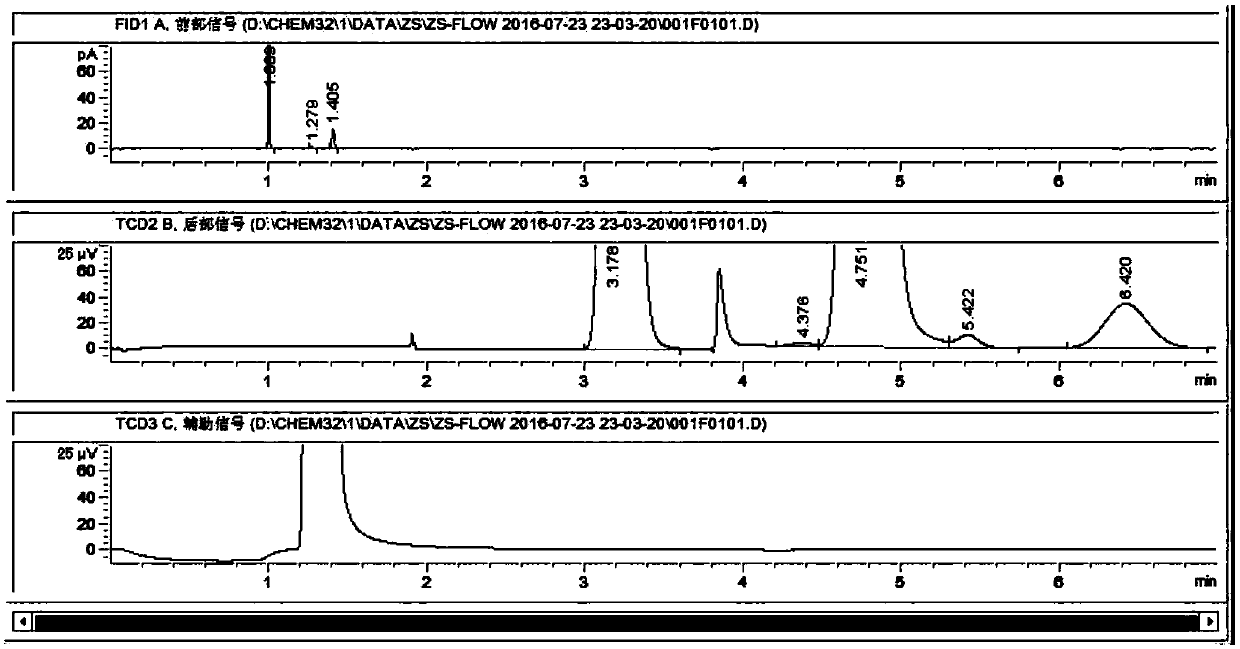
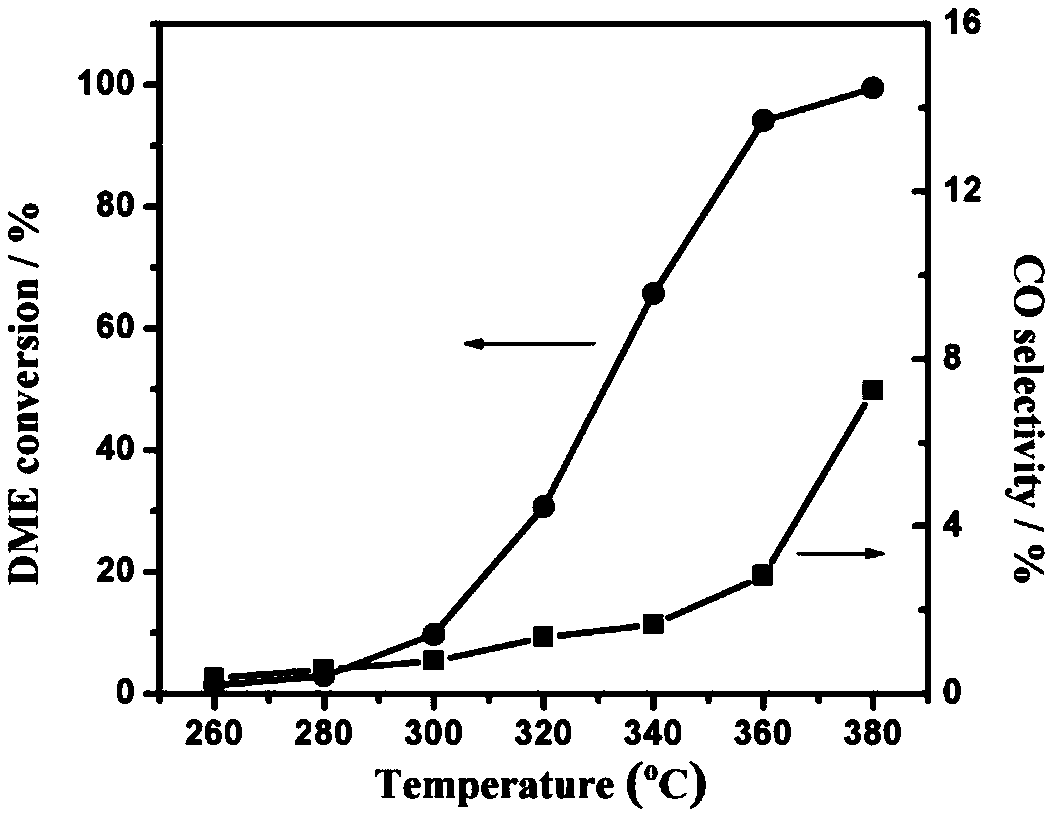
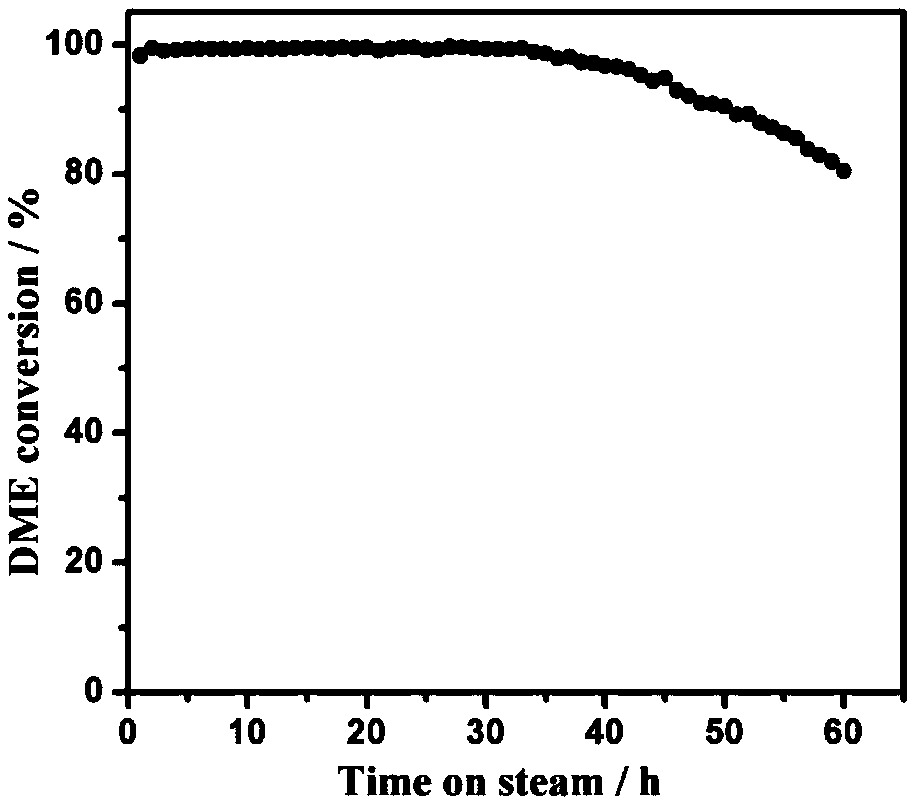
Zinc-modified copper-based catalyst for hydrogen production by steam reforming of dimethyl ether and preparation method thereof
A technology of steam reforming and copper-based catalysts, which is applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc. It can solve the problem that the preparation conditions are not easy to control and the catalyst is stable low catalytic activity and copper species are easy to be sintered and deactivated, and achieve broad industrial application prospects, excellent catalytic activity and selectivity, and novel preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1) 5.800g copper nitrate (Cu(NO 3 ) 2 ﹒ 3H 2 O) Dissolve in 80mL deionized water to prepare 0.3mol / L copper nitrate solution.
[0026] 2) At 20°C, add 25% ammonia water dropwise to the solution until the pH value of the solution is 11.8. Stir for 30 minutes to form a cuproammonia solution.
[0027] 3) 18.0 g of silica sol with a mass fraction of 25% was added dropwise to the above solution while stirring. After the dropwise addition, the suspension was sealed and mechanically stirred at 20° C. for 7 hours.
[0028] 4) Transfer the stirred suspension to an evaporator, magnetically stir in a 90°C water bath until the suspension becomes a thin paste, stop the water bath, cool to room temperature, and filter to obtain the product.
[0029] 5) The product was dried at 120° C. for 12 hours, and ground for later use.
[0030] 6) Take 1.735g zinc nitrate (Zn(NO 3 ) 2 ﹒ 6H 2 O) be dissolved in 100mL deionized water to obtain a zinc salt solution. The ground CuO-SiO 2...
Embodiment 2
[0034] According to the step of embodiment 1, the difference is that 1) get 6.940g zinc nitrate (Zn(NO 3 ) 2 ﹒ 6H 2 O) be dissolved in 100mL deionized water to obtain a zinc salt solution. The ground CuO-SiO 2 The sample was added to the solution and sonicated for 30 minutes to obtain a suspension.
Embodiment 3
[0036] According to the step of embodiment 1, the difference is that 1) get 3.470g zinc nitrate (Zn(NO 3 ) 2﹒ 6H 2 O) be dissolved in 100mL deionized water to obtain a zinc salt solution. The ground CuO-SiO 2 The sample was added to the solution and sonicated for 30 minutes to obtain a suspension.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com