Method of enriching and detecting miRNA in sample on the basis of magnetic bead coupled antibody, and matched microfluidic apparatus
A microfluidic device, a technology in samples, applied in chemical instruments and methods, biochemical equipment and methods, laboratory containers, etc., to achieve high-efficiency reaction and enrichment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
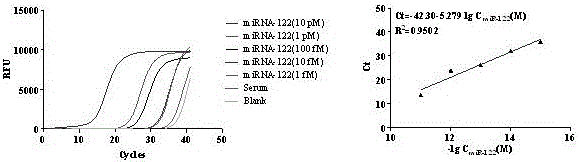
[0048] Example 1——enrichment of Ago2-miRNA complex in serum and detection of miRNA
[0049] 1 Preparation of immunomagnetic beads:
[0050] 1.1 Take 1.5 mg of Protein G magnetic beads and place them in a 2 mL centrifuge tube, use a magnetic separator to remove the original storage solution, and use 200 μL of PBS buffer to wash twice, and use a magnetic separator to remove the cleaning solution;
[0051] 1.2 Use 200 μL of PBS buffer to resuspend the magnetic beads, add 8 μg of anti-Ago2 rabbit monoclonal antibody, and incubate on an incubator at room temperature for 15 min;
[0052] 1.3 Wash the magnetic beads twice with 200 μL PBST buffer, shake gently for 1 min each time to prevent the beads from coagulating, remove excess antibody at the same time and transfer to a new centrifuge tube to obtain anti-Ago2 immunomagnetic beads.
[0053] 2. Immunomagnetic beads enrich the Ago2-miRNA complex in serum and detect miRNA (magnetic beads without antibody coupling are used as negative ...
Embodiment 2
[0061] Example 2—Microfluidic device enriches Ago2-miRNA complex in serum and detects miRNA
[0062] 1 The preparation of immunomagnetic beads is the same as in Example 1.
[0063] 2 Microfluidic device preparation:
[0064] 2.1 Design and prepare the microfluidic chip template according to the above magnetic bead immunoenrichment method;
[0065] 2.2 Pouring PDMS polymer on the microfluidic chip template and curing to obtain PDMS membrane;
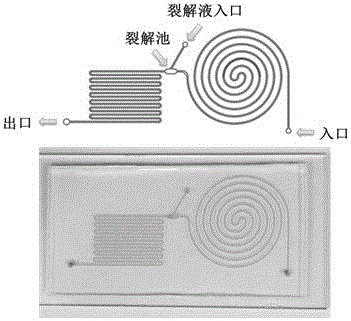
[0066] 2.3 Plasma bonding the PDMS membrane and the glass sheet, and packaging to obtain the required microfluidic chip device ( image 3 , Figure 4 ).
[0067] 3 Immunomagnetic beads were used to enrich Ago2-miRNA complexes in serum and detect miRNA on the microfluidic platform (the experiment was performed without antibody-coupled magnetic beads as a negative control)
[0068] 3.1 Pass 1 mL of BSA solution with a concentration of 1% through the microfluidic channel, the flow rate is controlled at 50 μL / min, and the microfluidic ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com