Method for purifying high-purity hydrogen or high-purity chlorosilane with phosphorus-containing impurities
A high-purity hydrogen and chlorosilane technology, used in halosilanes, halogenated silicon compounds, hydrogen separation, etc., can solve the problems of long total reflux time, increase operating costs, affect adsorption performance, etc., reduce impurity content, improve production quality, The effect of improving adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] This embodiment provides a method for purifying high-purity hydrogen with phosphorus-containing impurities, comprising the following steps:
[0026] Heating high-purity hydrogen with phosphorus-containing impurities to 500°C, the phosphorus-containing impurities in the high-purity hydrogen react with hydrogen to form phosphine, and then adsorb the phosphine through a non-loaded 4A molecular sieve adsorbent to obtain purified high-purity hydrogen.
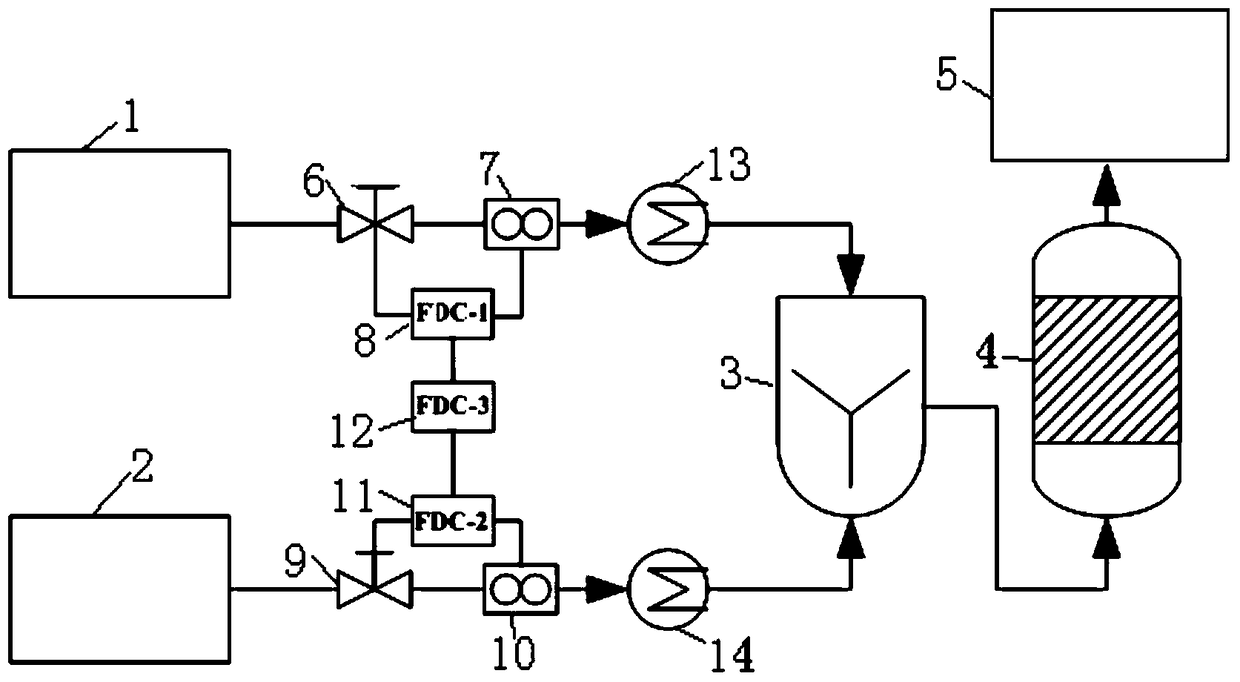
[0027] Such as figure 1 As shown, the present embodiment provides a device for purifying high-purity hydrogen with phosphorus-containing impurities, including:
[0028] High-purity hydrogen storage 1 containing phosphorus impurities, used to store high-purity hydrogen containing phosphorus impurities;
[0029] Chlorosilane reservoir 2, used to store chlorosilane;
[0030] A mixer 3 is connected to the high-purity hydrogen storage 1 containing phosphorus impurities and the chlorosilane storage 2 respectively, and the mixer 3...
Embodiment 2
[0041] This embodiment provides a method for purifying high-purity hydrogen with phosphorus-containing impurities, comprising the following steps:
[0042] Heating high-purity hydrogen with phosphorus-containing impurities to 500°C, the phosphorus-containing impurities in the high-purity hydrogen react with hydrogen to generate phosphine, and then adsorb the phosphine through a loaded 4A molecular sieve, and the loaded active ingredient is sodium chloride to obtain Purified high-purity hydrogen.
[0043] According to the same method as in Example 1, the purified high-purity hydrogen and chlorosilane in this example were passed into the reduction furnace to obtain polysilicon. The concentration of donor impurities in the polysilicon was 455.30ppta.
Embodiment 3
[0045] This embodiment provides a method for purifying high-purity hydrogen with phosphorus-containing impurities, comprising the following steps:
[0046] Heating high-purity hydrogen with phosphorus-containing impurities to 600°C, the phosphorus-containing impurities in the high-purity hydrogen react with hydrogen to generate phosphine, and then adsorb the phosphine through a non-loaded 5A molecular sieve to obtain purified high-purity hydrogen.
[0047] According to the same method as in Example 1, the purified high-purity hydrogen and chlorosilane in this example were passed into the reduction furnace to obtain polysilicon. The concentration of donor impurities in the polysilicon was 435.80ppta.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com