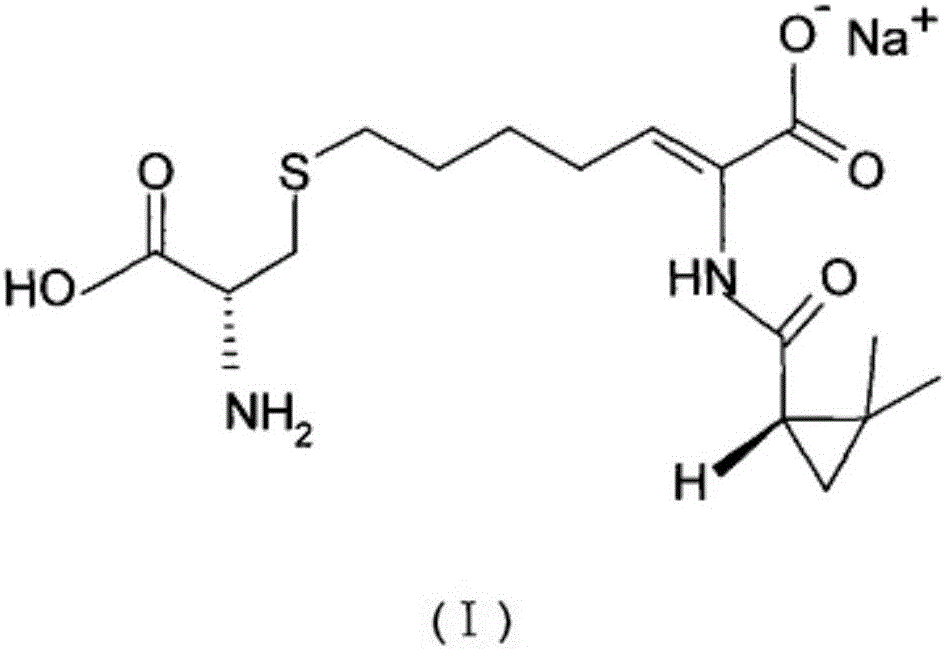
Preparation method of cilastatin sodium active pharmaceutical ingredient
A technology of cilastatin sodium and cilastatin, applied in the field of medicinal chemistry, can solve the problems of taking a long time, reducing the activity of raw materials, and taking a long time, avoiding the reduction of chemical activity and improving the quality and yield. , The effect of resin regeneration is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1: Preparation of cilastatin sodium of the present invention
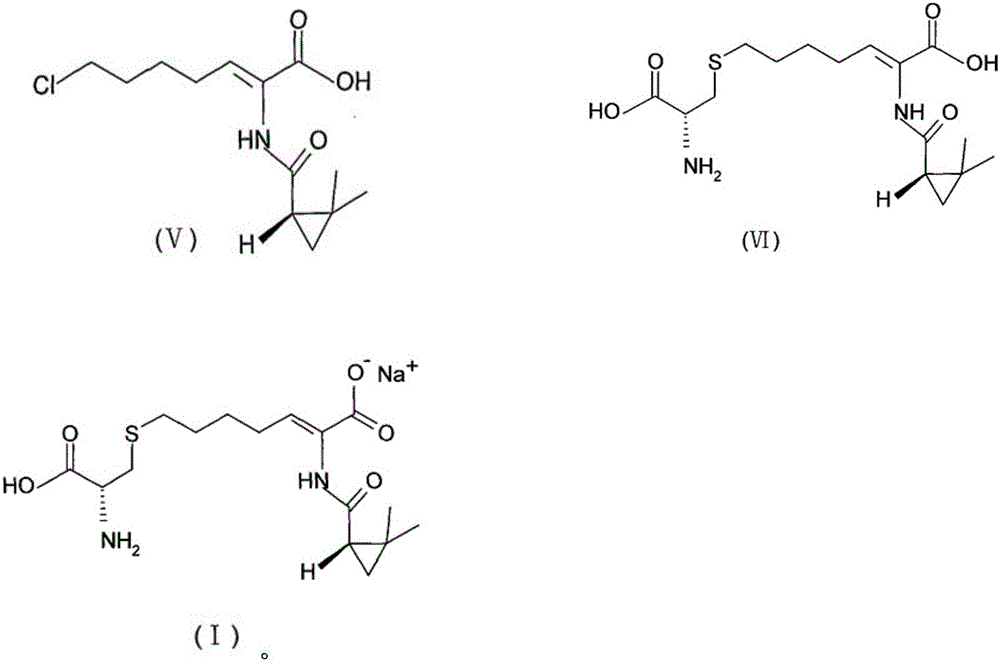
[0087] (1) Preparation of cilastatin compound represented by formula (VI):
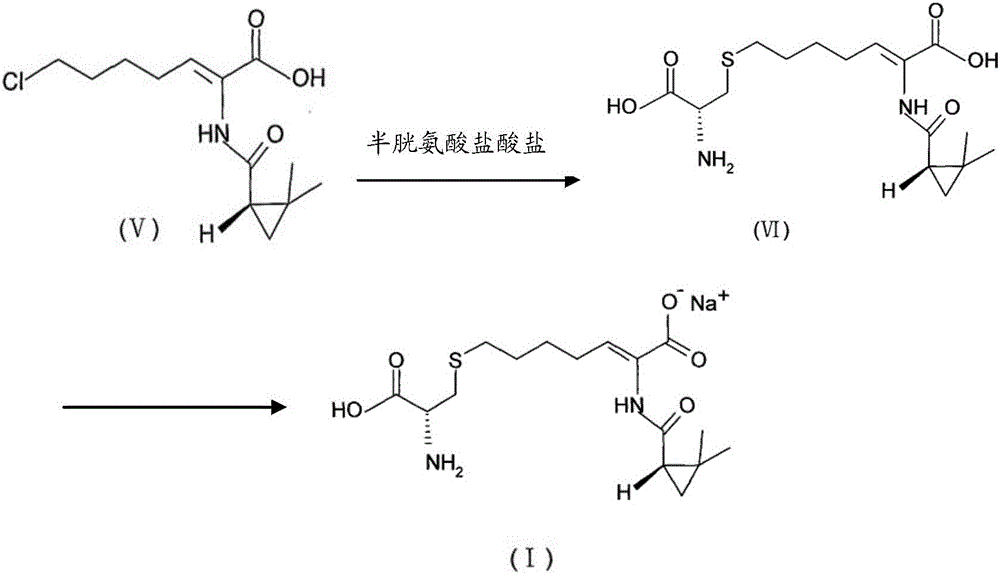
[0088] At 0°C, 15g sodium hydroxide was added to 100g water, stirred for 10min, and then 10g compound (Z)-7-chloro-2((S)-2,2-dimethyl ring represented by formula (V) was added Propylcarboxamido)-2-heptenoic acid, stir for 10 min; add 9 g of cysteine hydrochloride monohydrate at -5°C, stir for 10 min, and start the reaction at 25°C until it is analyzed by HPLC. The reaction is completed when the normalized content of (Z)-7-chloro-2((S)-2,2-dimethylcyclopropylcarboxamido)-2-heptenoic acid in the obtained reaction solution is less than 5% After cooling to 25°C, add 300g of water to the solution, stir, and slowly add 6M hydrochloric acid solution to the solution to adjust the pH to 1. The obtained solution was added to the Φ60*5.5m macroporous adsorption resin column HZ-820, first eluted with water, after the conductivity of the ...
Embodiment 2
[0091] Example 2: Preparation of cilastatin sodium of the present invention
[0092] (1) Preparation of cilastatin compound represented by formula (VI):
[0093] At 10°C, add 9g of sodium hydroxide to 80g of water, stir for 10min, and then add 10g of compound (Z)-7-chloro-2((S)-2,2-dimethyl ring represented by formula (V)) (Propylformamido)-2-heptenoic acid, stir for 10min; at 10℃, add 8.5g cysteine hydrochloride monohydrate, stir for 10min, the reaction starts at 35℃, until the HPLC analysis The reaction is completed when the normalized content of (Z)-7-chloro-2((S)-2,2-dimethylcyclopropylcarboxamido)-2-heptenoic acid in the obtained reaction solution is less than 5%; After cooling to 30°C, add 250 g of water to the solution, stir, and slowly add 6M hydrochloric acid solution to the solution to adjust the pH to 1.5. The obtained solution was added to the Φ35*3.5m macroporous adsorption resin column HZ-818, first eluted with water, after the conductivity of the collected solut...
Embodiment 3
[0096] Example 3: Preparation of cilastatin sodium of the present invention
[0097] (1) Preparation of cilastatin compound represented by formula (VI):
[0098] At 0℃, add 11g of sodium hydroxide to 70g of water, stir for 10min, and then add 10g of compound (Z)-7-chloro-2((S)-2,2-dimethyl ring represented by formula (V)) (Propylformamido)-2-heptenoic acid, stir for 10min; at 0℃, add 8.3g of cysteine hydrochloride monohydrate, stir for 10min, at 45℃, the reaction starts, until the HPLC analysis The reaction is completed when the normalized content of (Z)-7-chloro-2((S)-2,2-dimethylcyclopropylcarboxamido)-2-heptenoic acid in the obtained reaction solution is less than 5%; After cooling to 5°C, add 200 g of water to the solution, stir, and slowly add a 6M hydrochloric acid solution to the solution to adjust the pH to 2. The obtained solution was added to the Φ20*2.5m macroporous adsorption resin column HZ-816, and eluted with water first. After the conductivity of the collected ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com