Carbazole alkamine compound and preparation method thereof and application for parasitic disease resistance
A compound, carbazole technology, applied in the application field of anti-parasitic disease, can solve the problems of high cost, secondary infection, allergic reaction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
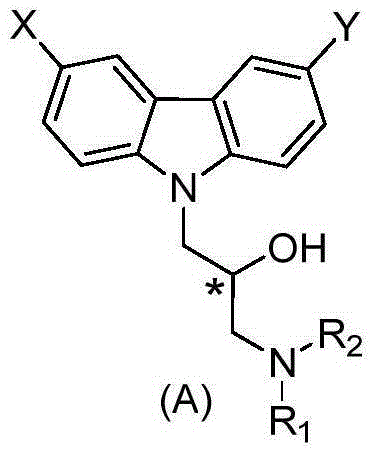
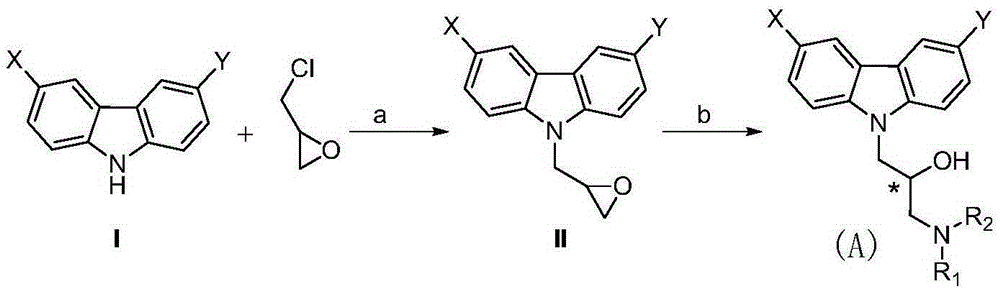
[0138] In the present invention, a preparation method of a compound of formula A comprises steps:
[0139]
[0140] (a) in an aprotic polar solvent, in the presence of a base, the substituted carbazole shown in formula I is reacted with epichlorohydrin to obtain a compound of formula II; and
[0141] (b) in a protic polar solvent, under Lewis acid catalysis, the formula II compound and HNR 1 R 2 Carry out the reaction, thereby obtain formula A compound;
[0142] In the above formulas, X, Y, R 1 and R 2 is defined as above.
[0143] In another preferred example, the method includes:
[0144] (1) In an aprotic polar solvent, under basic conditions, the substituted carbazole I is reacted with epichlorohydrin to obtain compound II, which is usually carried out with acetonitrile, acetone, dimethyl sulfoxide, dimethyl Formamide, etc. are used as solvents, and the bases used are triethylamine, diethylamine, pyridine, cesium carbonate, lithium hydroxide, potassium hydroxide, ...
Embodiment 1
[0182] Embodiment 1, 3,6-dichloro-9-epoxypropyl carbazole (IIa):
[0183] 3,6-Dichlorocarbazole (470mg, 2mmol) was dissolved in 20mL DMF, and potassium hydroxide (135mg, 2.4mmol) was slowly added under ice-bath conditions, and stirred for 0.5-1 hour after the addition, until the potassium hydroxide was solid completely dissolved.
[0184] Then epichlorohydrin (2.4 mmol) was slowly added dropwise into the system, and reacted for 4-5 hours. After the reaction, add 30ml of water, extract with ethyl acetate (30ml×3), wash with saturated sodium chloride solution (30ml×3), dry the organic layer with anhydrous sodium sulfate, filter, and recover the solvent from the filtrate under reduced pressure, the crude product Purified by column chromatography to obtain 293 mg of white solid, yield 50%.
Embodiment 2
[0185] Embodiment 2, 9-epoxypropyl carbazole (IIb):
[0186] Refer to Example 1 for the operation process, and replace 3,6-dichlorocarbazole with carbazole to obtain a white solid with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com