Carbonyl conjugated heterocyclic compound and preparation and application
A technology for conjugated heterocycles and compounds is applied in the fields of carbonyl-conjugated heterocycles and their preparation and application, can solve problems such as high energy consumption for a long time, and achieves a simple and feasible synthesis method, high yield, and avoids processing steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
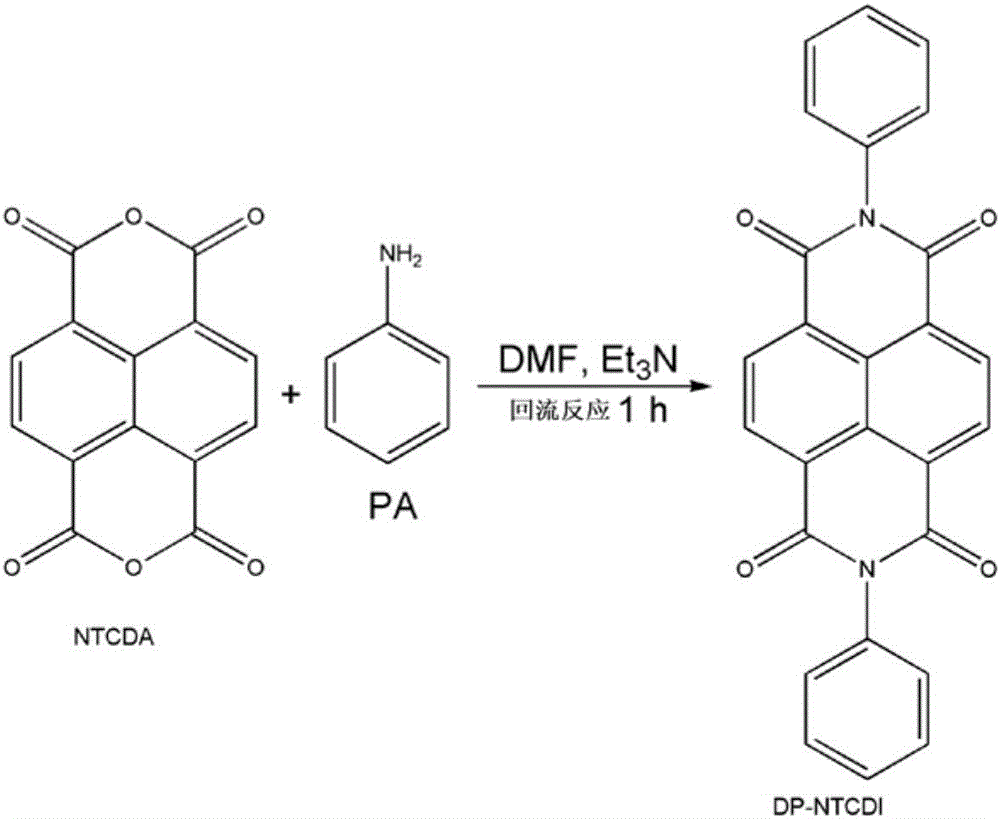
[0028] (1) Dissolve 2g of 1,4,5,8-naphthalenetetracarboxylic anhydride (NTCDA, 7.6mmol) in 40.0mL dimethylformamide (DMF) and magnetically stir at 130°C;
[0029] (2) Then 2.1mL of aniline (PA, 23.0mmol) and 4.3mL of triethylamine (30.0mmol) were dropped into the solution of step (1) dropwise, followed by heating to reflux under magnetic stirring for 1 hour and brown Precipitation;
[0030] (3) The mixed solution obtained in step (2) is removed by centrifugation and filtration to obtain a brown precipitate;
[0031] (4) Dispersing the obtained brown precipitate in ethanol, and thoroughly washing the soluble impurities with ethanol and then filtering to obtain the crude product;
[0032] (5) The product obtained in step (4) is then dissolved in dimethylformamide (DMF) and recrystallized to obtain an orange needle-shaped recrystallized product;
[0033] (6) washing and filtering the recrystallized product obtained in step (5) with ethanol once;
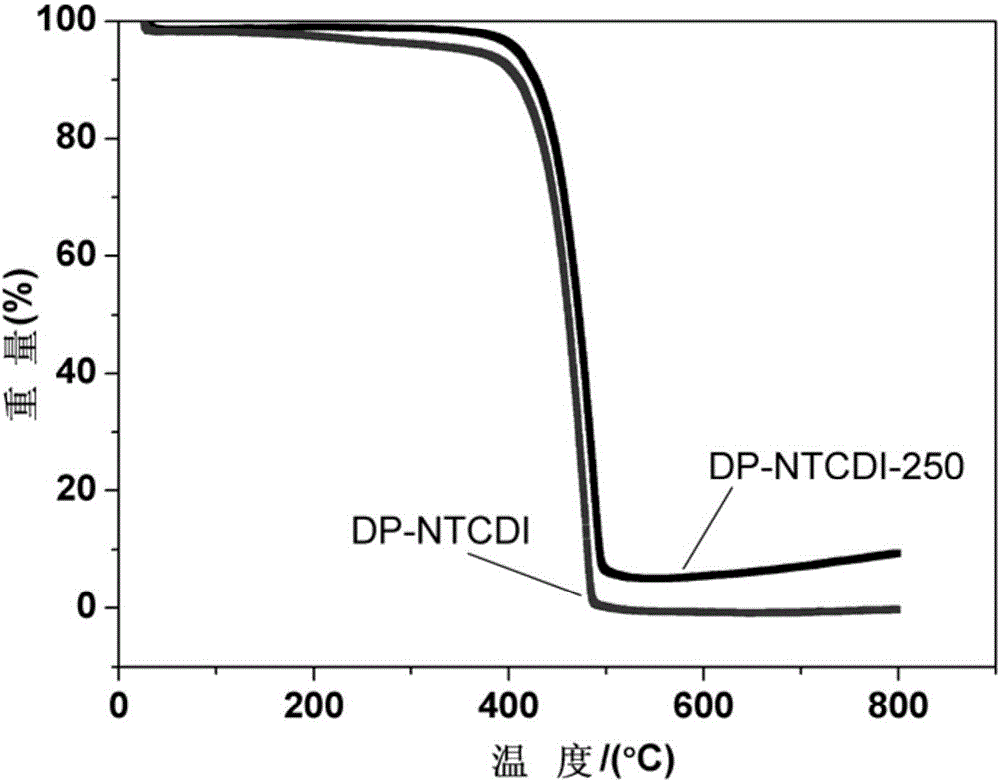
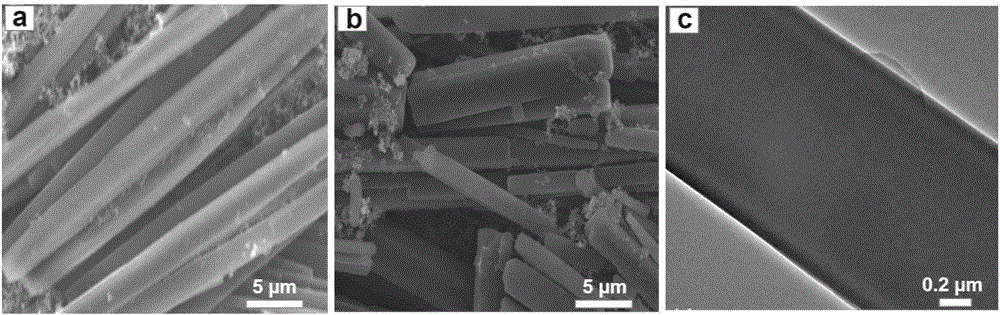
[0034] (7) The product obtain...
Embodiment 2
[0044] (1) Dissolve 2g of 1,4,5,8-naphthalenetetracarboxylic anhydride (NTCDA, 7.6mmol) in 40.0mL acetone and magnetically stir at 50°C;
[0045] (2) Then 2.1mL of aniline (PA, 23.0mmol) and 4.3mL of triethylamine (30.0mmol) were dropped into the solution of step (1) dropwise, and then heated under magnetic stirring to reflux for 0.5 hours and then brown Precipitation;
[0046] (3) The mixed solution obtained in step (2) is removed by centrifugation and filtration to obtain a brown precipitate;
[0047] (4) Dispersing the obtained brown precipitate in ethanol, and thoroughly washing the soluble impurities with ethanol and then filtering to obtain the crude product;
[0048] (5) The product obtained in step (4) is then dissolved in dimethylformamide (DMF) and recrystallized to obtain an orange needle-shaped recrystallized product;
[0049] (6) washing and filtering the recrystallized product obtained in step (5) with deionized water once;
[0050] (7) The product obtained in...
Embodiment 3
[0054] (1) Dissolve 2g of 1,4,5,8-naphthalenetetracarboxylic anhydride (NTCDA, 7.6mmol) in 40.0mL of methanol and magnetically stir at 60°C;
[0055] (2) Then 2.1mL of aniline (PA, 23.0mmol) and 4.3mL of triethylamine (30.0mmol) were dropped into the solution of step (1) dropwise, followed by heating to reflux under magnetic stirring for 2 hours and brown Precipitation;
[0056] (3) The mixed solution obtained in step (2) is removed by centrifugation and filtration to obtain a brown precipitate;
[0057] (4) Dispersing the obtained brown precipitate in ethanol, and thoroughly washing the soluble impurities with ethanol and then filtering to obtain the crude product;
[0058] (5) The product obtained in step (4) is then dissolved in dimethylformamide (DMF) and recrystallized to obtain an orange needle-shaped recrystallized product;
[0059] (6) washing and filtering the recrystallized product obtained in step (5) with ethanol once;
[0060] (7) The product obtained in step (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com