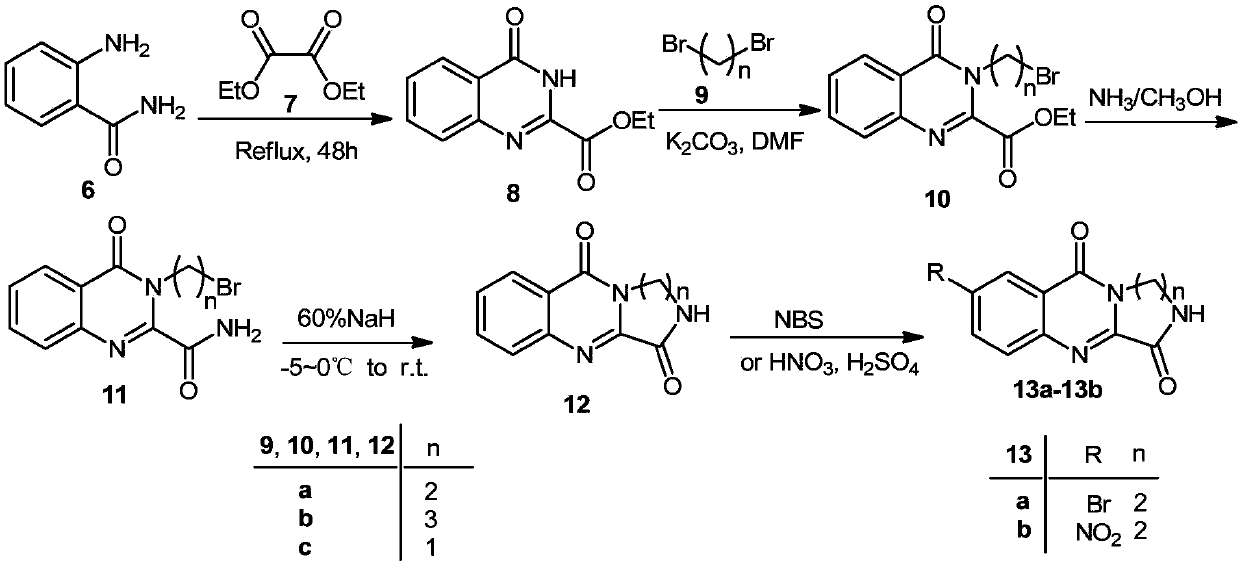
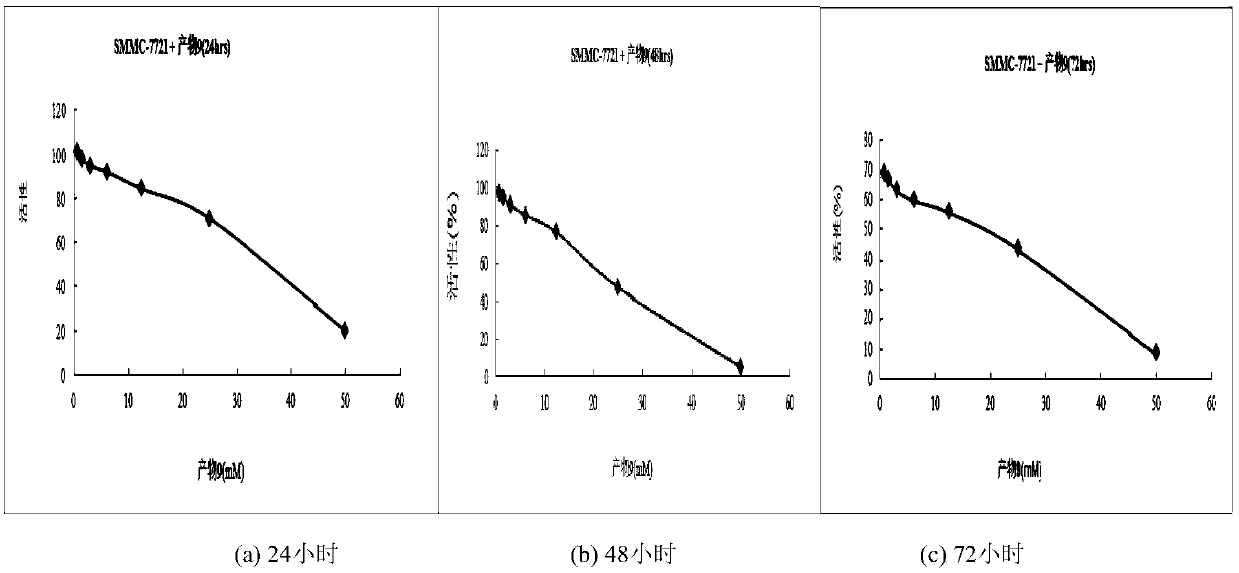
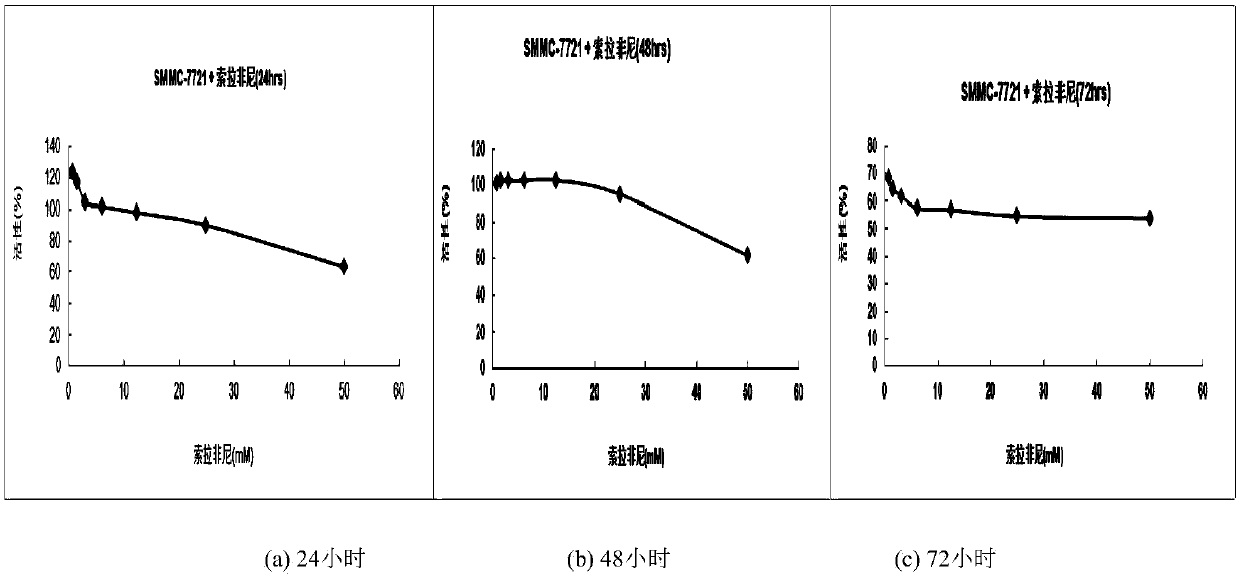
A class of 2,3-lactam ring-fused quinazolin-4(3h)-one derivatives and its preparation method and application
A technology of ring-fused quinazoline and lactam, which is applied in the field of pharmaceutical preparation, can solve the problem of not many researches on combining and combining, and achieves the effects of simple post-processing, easy operation and mild conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: Synthesis of 4-oxo-3,4-dihydroquinazoline-2-carboxylic acid ethyl ester (8)
[0064] Add anthranilamide (6, 6.8g, 50mmol), diethyl oxalate (7, 50mL, 367mmol) and a magneton into a 250ml three-necked flask, heat and stir, and the temperature is 140°C at this time, by clarification The light yellow color of the reaction solution changed to turbid orange red, and reflux began at 150° C. after one hour, and turned yellowish white after five hours. After 48 hours, the product showed no fluorescence, indicating that the reaction was complete. After cooling to room temperature, a solid precipitated out. Filter with suction and wash with absolute ethanol to obtain a filter cake. The obtained filter cake was recrystallized with absolute ethanol, and the obtained product was dried under vacuum to obtain the desired intermediate 8 (4.928 g, 44.8%) as fine white needle-like crystals, m.p.185.8-187.4°C.
Embodiment 2
[0065] Embodiment 2: Synthesis of 4-oxo-3,4-dihydroquinazoline-2-carboxylic acid ethyl ester (8)
[0066] Diethyl oxalate (7, 50 mL, 0.366 mol) was slowly and carefully added to melted 2-aminobenzamide (6, 6.8 g, 0.05 mol) in portions. The reaction mixture was stirred and heated to reflux for 48 hours, followed by thin layer chromatography (TLC) monitoring (developing solvent: DCM-Et 2O, v:v=1:1) reaction process. After confirming that the reaction was complete, the reaction liquid was cooled to room temperature, and excess diethyl oxalate was distilled off under reduced pressure. The obtained solid was washed with cold ethanol (100 mL), and the filter cake obtained after suction filtration was resuspended in cold diethyl ether (50 mL), stirred for 0.5 hours, and filtered to obtain the desired intermediate (8, 8.6 g, 78.9%) as fine white Needle-like crystals, m.p.190–191°C.
Embodiment 3
[0067] Example 3: Synthesis of 4-oxo-3,4-dihydro-quinazoline-2-carboxylic acid ethyl ester (8)
[0068] Set up the reflux device, add anthranilamide (6, 6.8g, 50mmol) and diethyl oxalate (7, 50mL, 367mmol) and magneton into a 250mL three-necked bottle, heat and stir, and the temperature is 140 °C, from clear light yellow to turbid orange red, reflux at 150 °C after one hour, and turn into yellowish white after 5 hours, TLC monitoring after 48 hours of reaction, the product has no fluorescence or fluorescent spots, indicating that the reaction is complete . After cooling to room temperature, a solid precipitated out. The filter cake was obtained by suction filtration and washed with absolute ethanol. Recrystallized from absolute ethanol and dried in vacuo to obtain the desired intermediate product (8, 5.84g, 53.1%), m.p.186-187°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com