Preparation method of unsaturated hyaluronic acid odd oligosaccharides
A technology of hyaluronic acid and hyaluronidase, applied in the field of biomedicine, can solve the problems of low reaction efficiency, complex process, non-single reaction product structure, etc., and achieve the effects of single structure, simplified preparation process and improved preparation efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Step 1) Prepare the hyaluronic acid (HA) solution with a concentration of 10 mg / mL from the lyophilized powdered hyaluronic acid (HA) with pure water, and prepare the lyophilized powdered microbial hyaluronidase with an enzymatic hydrolysis working solution 200IU / mL hyaluronidase solution;
[0038] Further, the enzymolysis working solution is pure water;
[0039] Further, the microbial hyaluronidase is derived from Streptomyces hyalurolyticus;
[0040] Step 2) Take 5 parts of the hyaluronic acid (HA) solution, add 1 part of the hyaluronidase solution under ice bath conditions, shake and mix, and incubate in a 37°C water bath for 24 hours to prepare a sample; The sample was moved to a water bath at 100°C, heated for 5 minutes, centrifuged, and precipitated;
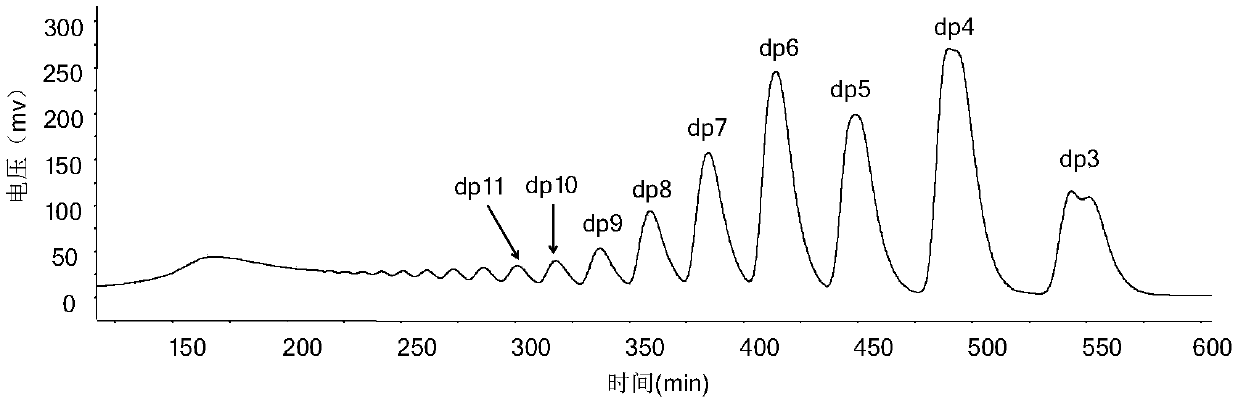
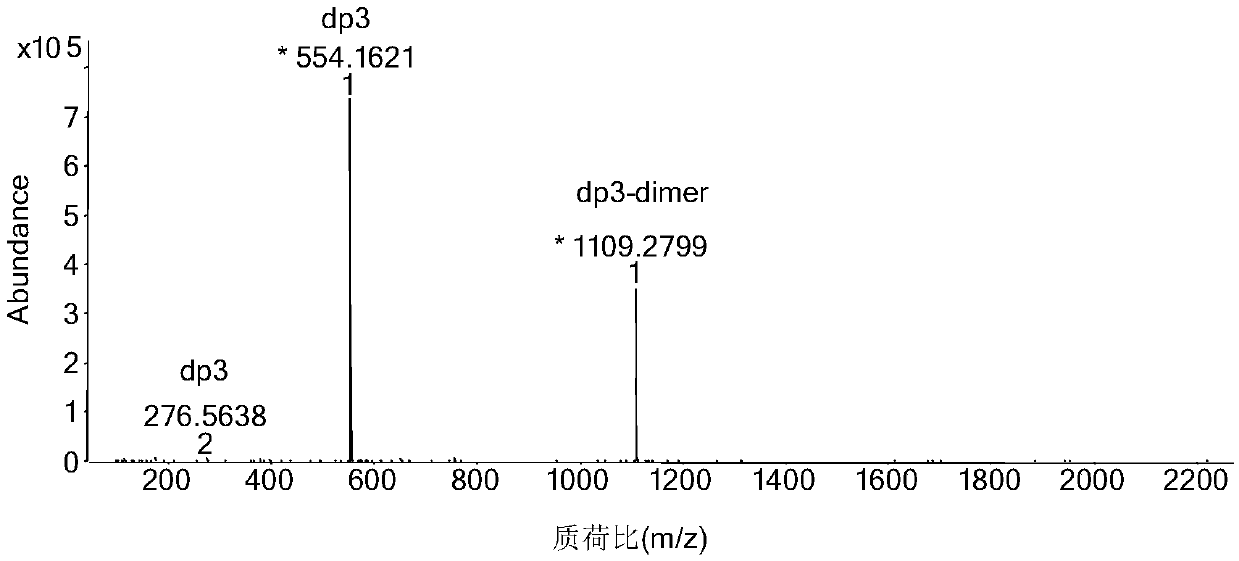
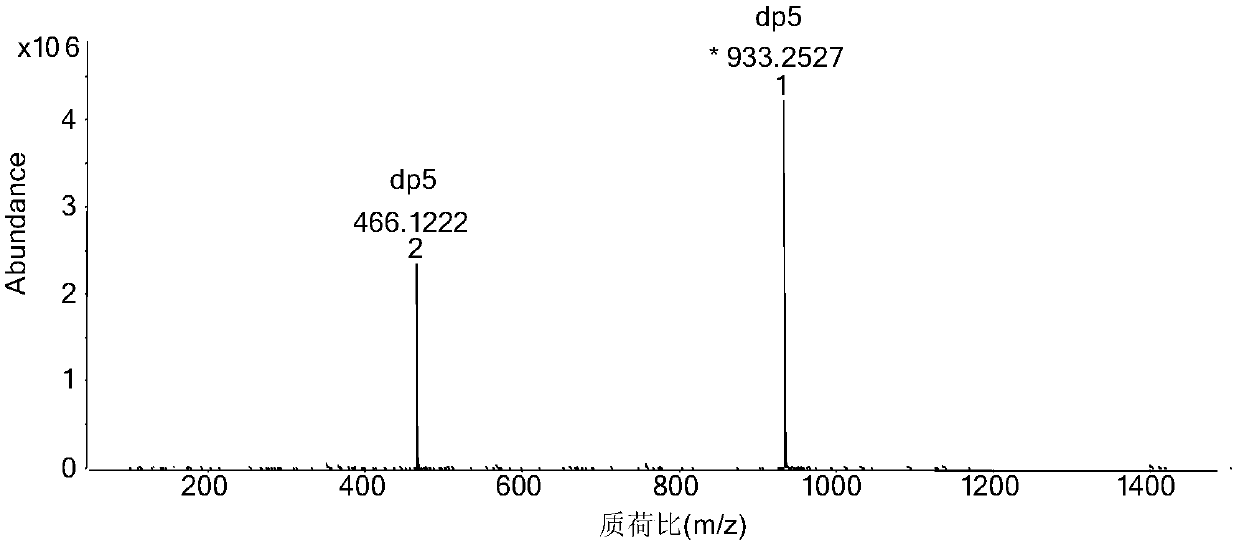
[0041] Step 3) using a low-pressure chromatography column (2.6 × 100cm) with a filler of Bio-gel P10 to separate and purify the sample after deprecipitation, wherein the mobile phase concentration is 0.2M sodium chl...
Embodiment 2
[0044] Step 1) Prepare lyophilized powdered hyaluronic acid (HA) with pure water to form a hyaluronic acid (HA) solution with a concentration of 8 mg / mL, and prepare lyophilized powdered microbial hyaluronidase with enzymatic hydrolysis working solution 150IU / mL hyaluronidase solution;
[0045] Further, the enzymolysis working solution is pure water;
[0046] Further, the microbial hyaluronidase is derived from Streptomyces hyalurolyticus;
[0047] Step 2) Take 8 parts of the hyaluronic acid (HA) solution, add 2 parts of the hyaluronidase solution under ice bath conditions, shake and mix, and incubate in a 37°C water bath for 30 hours to prepare a sample; The sample was moved to a water bath at 100°C, heated for 7 minutes, centrifuged, and precipitated;
[0048] Step 3) using a low-pressure chromatography column (2.6 × 100cm) filled with Bio-gel P6 to separate and purify the sample after deprecipitation, wherein the mobile phase concentration is 0.1M potassium chloride, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com