Novel cyclic phospholipids used for treating HCV infection
A therapeutic agent, a technology of phosphorus dioxide, applied in the field of novel cyclophosphate, to achieve the effects of improving pharmacokinetic properties, reducing toxicity and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
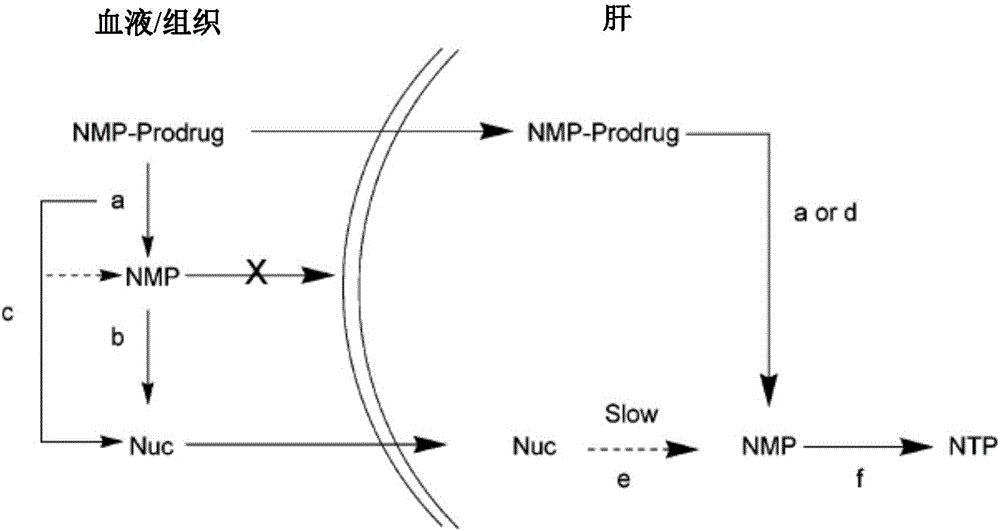
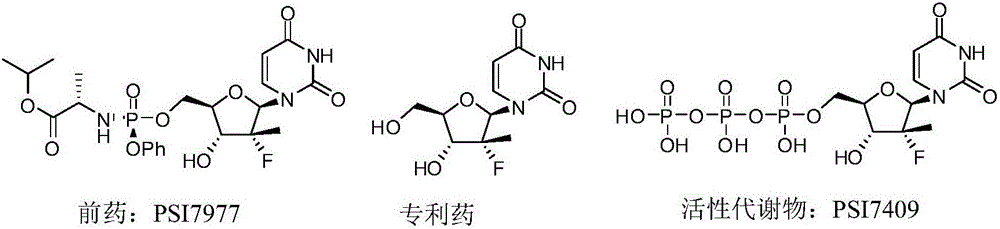
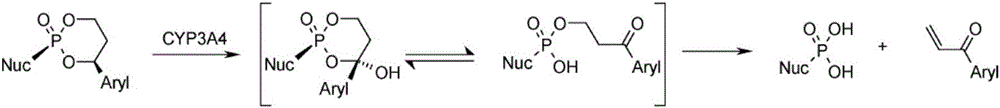
Image
Examples
Embodiment 1
[0064] Example 1. Preparation of 1-((2R,3R,4R,5R)-5-(((4-(benzo[d][1,3]dioxa-5-position)-2-oxidation-1 ,3,2-dioxaphosphorinane-2-position)oxy)methyl)-3-fluoro-4-hydroxy-3-methyltetrahydrofuran-2-position)pyrimidine-2,4(1H,3H )-diketone (5)
[0065]
[0066] Compound 5 was prepared according to the following process:
[0067]
[0068] Compound 1 (17.000g, 0.103mol) was dissolved in toluene (150ml), N 2 Under protection, sodium hydrogen (8.284g, 0.207mol, 2eq) was added in batches in an ice bath. After the addition was completed, the stirring reaction was continued for 30 min, and then the compound dimethyl carbonate (27.834 g, 0.309 mol, 3.0 eq) was slowly added dropwise. After the drop, the temperature was raised to reflux for 3 hours. TLC monitored the completion of the reaction. The reaction solution was poured into saturated ammonium chloride solution, extracted with ethyl acetate (150ml*3), the organic phase was separated, and washed 2-3 times with saturated brine...
Embodiment 2
[0072] Example 2. Preparation of cis 1-((2R,3R,4R,5R)-5-(((4-(benzo[d][1,3]dioxa-5-position)-2-oxidation- 1,3,2-Dioxaphosphorinane-2-position)oxy)methyl)-3-fluoro-4-hydroxy-3-methyltetrahydrofuran-2-position)pyrimidine-2,4(1H, 3H)-Diketone (6)
[0073]
[0074] Compound 6 was prepared according to the following process:
[0075]
[0076] Compound 4 (3.140g, 0.016mol) was dissolved in anhydrous THF (50ml), N 2 Triethylamine (6.464 g, 0.064 mol) was added in an ice bath under protection. A THF solution (10 ml) of 4-nitrophenyl phosphate dichloride (4.300 g, 0.0168 mol) was then added. After the dropwise addition, the mixture was raised to room temperature and stirred for 1 hour, then refluxed and stirred for 2 hours. Cool to 30°C, add sodium 4-nitrophenolate (7.728g, 0.048mol) and continue to reflux for 2 hours, then overnight at room temperature. The reaction was quenched with saturated ammonium chloride, extracted with ethyl acetate, and the filtrate was spin-dried ...
Embodiment 3
[0078] Example 3. Preparation of cis 1-((2R,3R,4R,5R)-5-((((2R,4S)-4-(benzo[d][1,3]dioxa-5-position )-2-oxidation-1,3,2-dioxaphosphorinane-2-position)oxy)methyl)-3-fluoro-4-hydroxyl-3-methyltetrahydrofuran-2-position)pyrimidine- 2,4(1H,3H)-Diketone (7)
[0079]
[0080] Compound 7 was prepared according to the following scheme:
[0081]
[0082] Triethylamine (2.659g, 0.026mol) was slowly added dropwise into HCOOH (3.981g, 0.089mol), and the temperature was controlled below 40°C. After the addition, compound 2 (15.000g, 0.068mol) was dissolved in DMF ( 50ml), nitrogen bubbling to remove oxygen in the system, then add catalyst (s,s)-Ts-DPEN-RuCl-(p-cymene) (0.042g, 0.068mmol), heat to 60°C, react for 19h, TLC shows the reaction up about 1 / 3. Add catalyst (0.042g, 0.068mmol), then react for 5 hours, pour the reaction solution into water, extract 3 times with ethyl acetate (100ml), combine the ethyl acetate layer, wash 2 times with water, and wash with saturated brine (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com