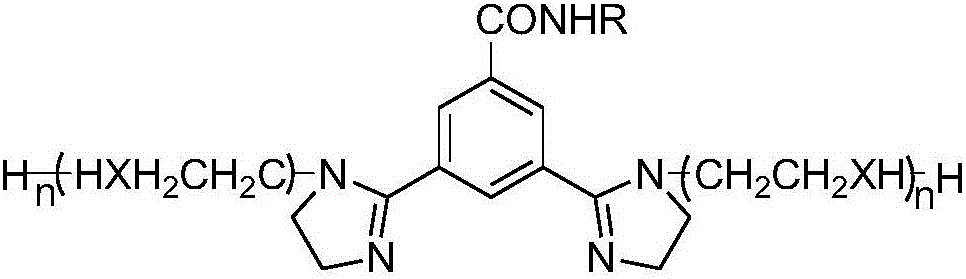
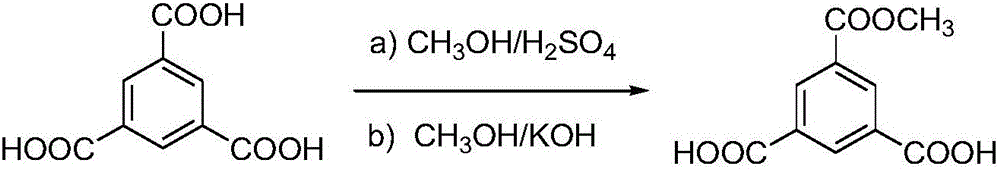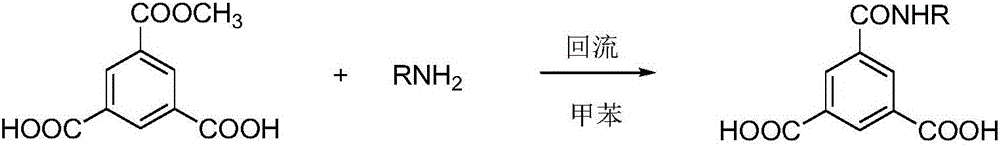Adsorbed film type imidazoline corrosion inhibitor and preparing method thereof
An imidazoline corrosion inhibitor, film-forming technology, applied in the direction of organic chemistry, can solve the problems of corrosion inhibitor shedding, reduced adsorption film formation efficiency, shortened corrosion inhibitor film life, etc., to achieve low dosage of chemicals, adsorption Stable and firm effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Synthesis of 3,5-dicarboxylic acid-1-methyl benzoate
[0029] a) Accurately weigh trimesic acid sequentially into a four-neck flask equipped with a thermometer, agitator, condenser and water separator, use excess methanol as a solvent, and then add concentrated H 2 SO 4 , wherein the mass ratio of trimesic acid and methanol is 1:2, trimesic acid and concentrated H 2 SO 4 Molar ratio 1:0.04, reflux for 9h, add Na 2 CO 3 to neutral, extracted with ethyl acetate, MgSO 4 Dry, filter and distill off the solvent to obtain trimethyl trimesic acid.
[0030] b) Dissolve trimethyl trimesate in methanol, the mass ratio of trimethyl trimesate to methanol is 1:25, add KOH in batches under stirring in an ice-salt bath, trimethyl trimesate and KOH molar ratio of 1:2, stirred at 0°C for 2 hours, naturally heated to 25°C, distilled off the solvent methanol, added ethyl acetate, washed, filtered to obtain potassium salt, dissolved in water, acidified with concentrated HCl to pH...
Embodiment 2
[0036] (1) Synthesis of 3,5-dicarboxylic acid-1-methyl benzoate
[0037] a) Accurately weigh trimesic acid sequentially into a four-neck flask equipped with a thermometer, agitator, condenser and water separator, use excess methanol as a solvent, and then add concentrated H 2 SO 4 , wherein the mass ratio of trimesic acid and methanol is 1:5, trimesic acid and concentrated H 2 SO 4 Molar ratio 1:0.03, reflux for 12h, add Na 2 CO 3 to neutral, extracted with ethyl acetate, MgSO 4 Dry, filter and distill off the solvent, trimethyl trimesate.
[0038] b) Put trimethyl trimesate in methanol, the mass ratio of trimethyl trimesate to methanol is 1:10, add KOH in batches under stirring in an ice-salt bath, the ratio of trimethyl trimesate to KOH Molar ratio 1:2, stirred at 0°C for 2 hours, naturally heated to 25°C, distilled off the solvent methanol, added ethyl acetate, washed, filtered to obtain potassium salt, dissolved in water, acidified with concentrated HCl to pH 3, extr...
Embodiment 3
[0044] (1) Synthesis of 3,5-dicarboxylic acid-1-methyl benzoate
[0045] a) Accurately weigh trimesic acid sequentially into a four-neck flask equipped with a thermometer, agitator, condenser and water separator, use excess methanol as a solvent, and then add concentrated H 2 SO 4 , wherein the mass ratio of trimesic acid and methanol is 1:5, trimesic acid and concentrated H 2 SO 4 Molar ratio 1:0.03, reflux for 12h, add Na 2 CO 3 to neutral, extracted with ethyl acetate, MgSO 4 Dry, filter and distill off the solvent, trimethyl trimesate.
[0046] b) Put trimethyl trimesate in methanol, the mass ratio of trimethyl trimesate to methanol is 1:10, add KOH in batches under stirring in an ice-salt bath, the ratio of trimethyl trimesate to KOH Molar ratio 1:2, stirred at 0°C for 2 hours, naturally heated to 25°C, evaporated solvent methanol, added ethyl acetate, washed, filtered to obtain potassium salt, dissolved in water, acidified with concentrated HCl to pH 3, extracted w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com