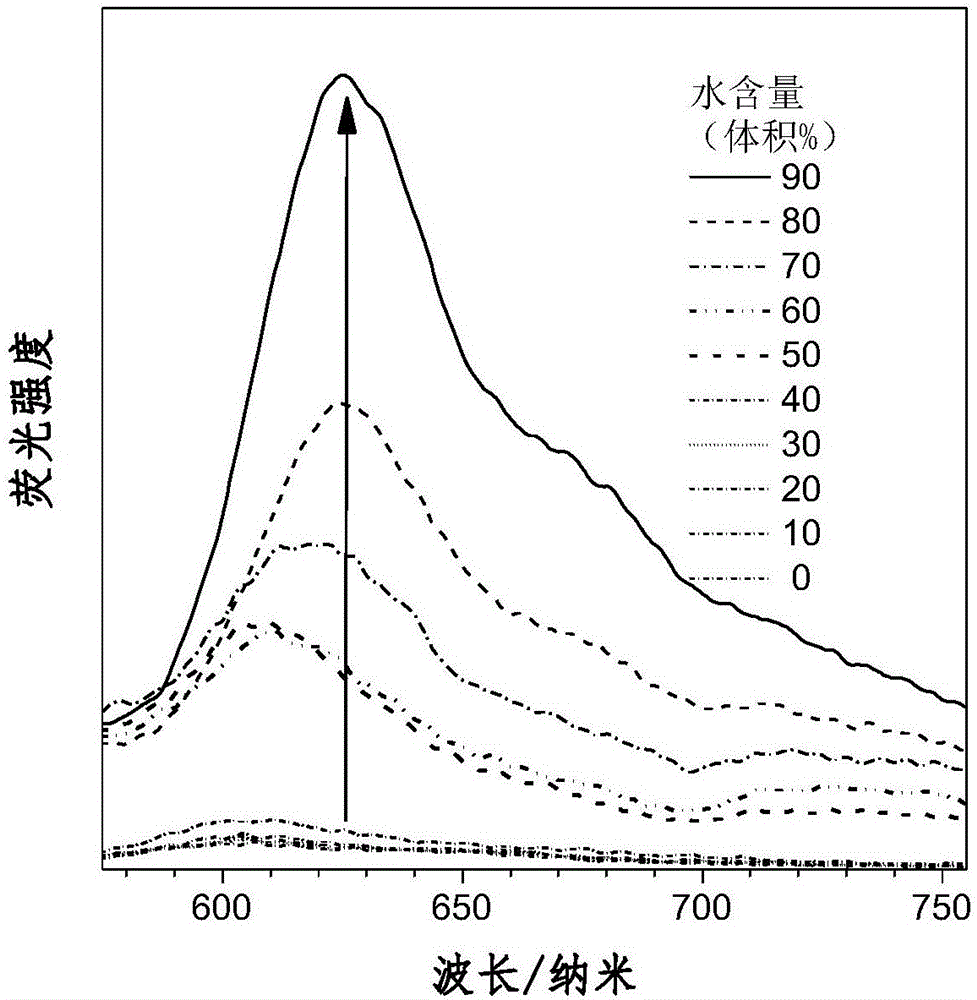
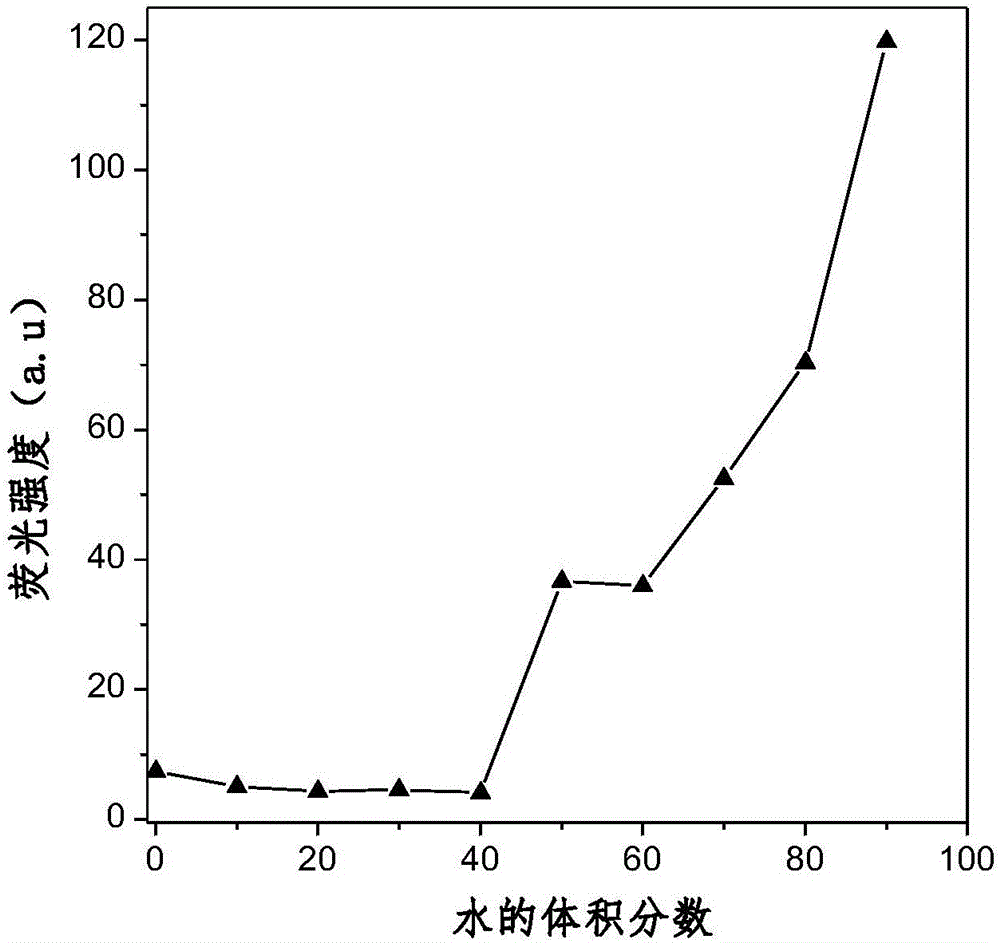
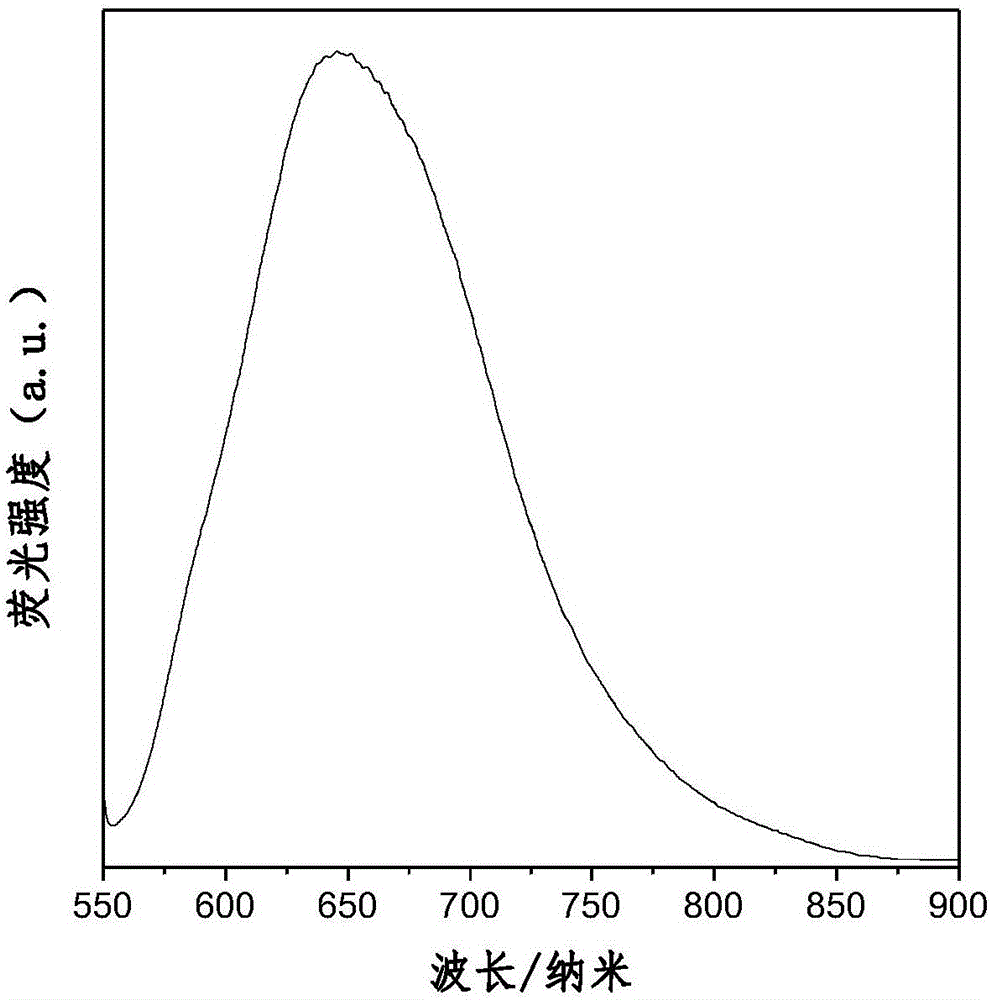
Aggregation-induced red light emission material and preparation method thereof
An aggregation-inducing and red-emitting technology, which is applied in the direction of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems that the final product is not 100% pure and difficult to separate, and achieves mild reaction conditions, easy purification, Experimental effect of cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] In a preferred technical solution of the present invention, R 1 is n-butyl, R 2 for -H.
[0053] (1) Synthesis of intermediate 1-(4-hydroxyphenyl)-1,2,2-triphenylethylene. 4-Hydroxybenzophenone (1.9 g, 10 mmol), benzophenone (2.2 g, 12 mmol), and Zn powder (2.9 g, 44 mmol) were added to a 250 mL round bottom flask. After vacuuming and changing nitrogen three times, 80 mL of tetrahydrofuran was added. After cooling to 0°C, slowly add TiCl 4 (4.2 g, 22 mmol), followed by stirring for 1 h. Then stir at 70°C for 24h, after cooling to room temperature, add 80mL of dilute hydrochloric acid (1mol / L) to adjust to neutrality, extract three times with DCM, collect the organic layer, dry over anhydrous magnesium sulfate, and spin the solvent to obtain the crude product. A 20:1 mixture of petroleum ether and ethyl acetate as eluent, SiO 2 As a stationary phase, 0.8 g of a white solid was obtained by column chromatography, and the yield was 47%.
[0054]
[0055] (2) Synth...
Embodiment 2
[0063] In another preferred technical solution of the present invention, R 1 is cyclohexyl, R 2 for -CH 3 .
[0064] (1) Synthesis of intermediate 4-(1-phenyl-2,2-xylylvinyl)phenol. 4-Hydroxybenzophenone (1.9g, 10mmol), 4,4'-dimethylbenzophenone (3.2g, 15mmol), and Zn powder (3.9g, 60mmol) were added to a 250mL round bottom flask. After vacuuming and changing nitrogen three times, 80 mL of tetrahydrofuran was added. After cooling to 0°C, slowly add TiCl 4 (5.7g, 30mmol), followed by stirring for 0.8h. Then stir at 65°C for 36h, after cooling to room temperature, add 80mL of dilute hydrochloric acid (1mol / L) to adjust to neutrality, extract three times with DCM, collect the organic layer, dry over anhydrous magnesium sulfate, and spin the solvent to obtain the crude product. A 40:1 mixture of petroleum ether and ethyl acetate as eluent, SiO 2 As a stationary phase, 0.62 g of a white solid was obtained by column chromatography, with a yield of 32%.
[0065]
[0066] (...
Embodiment 3
[0072] In another preferred technical solution of the present invention, R 1 is n-octyl, R 2 for-OC 2 h 5 .
[0073] (1) Synthesis of intermediate 4,4'-diethoxybenzophenone. Add bromoethane (8.1g, 75mmol) and 4,4'-dihydroxybenzophenone (5.4g, 25mmol) into a three-necked flask, add 200mL of acetone, and then add potassium carbonate (10.4g, 75mmol), Then the temperature was raised to 55°C and stirring was continued for 36h. After the reaction stopped, cool to room temperature, filter, collect the filtrate, and dry the solvent by rotary evaporation to obtain the crude product, using a mixture of petroleum ether and ethyl acetate with a volume ratio of 40:1 as the eluent, SiO 2 As a stationary phase, 5.5 g of 4,4'-diethoxybenzophenone was obtained after separation and purification by column chromatography, with a yield of 81%.
[0074]
[0075] (2) Synthesis of intermediate 4-(2,2-bis(4-ethoxyphenyl)-1-phenylvinyl)phenol. Into a 250 mL round bottom flask were added 4-hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com