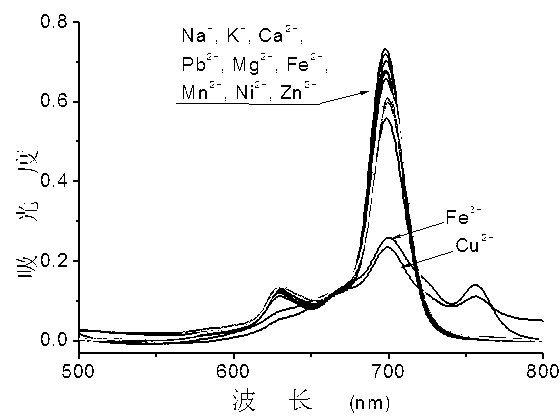
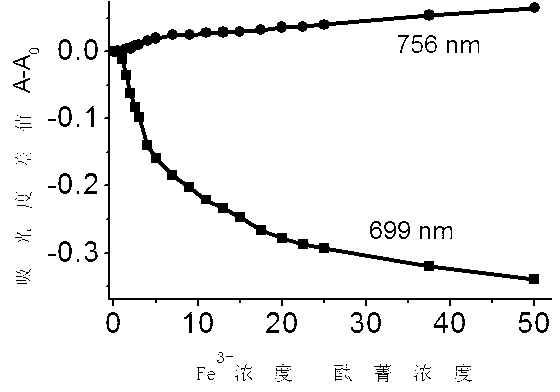
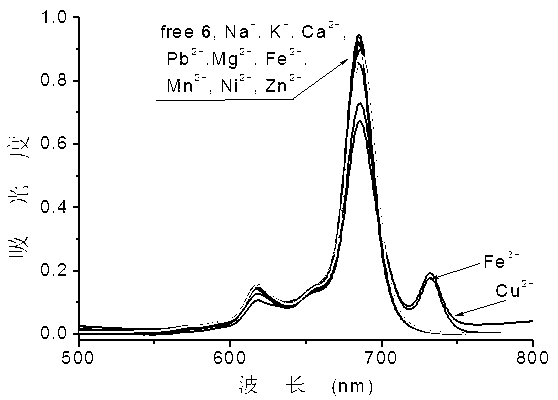
Ortho-positioned bridged azacrown ether modified phthalocyanin and preparation method and application thereof
A technology of heterocrown ether and azine, applied in the field of preparation of optical functional substances, can solve the problems of high absorption and emission intensity, lack of phthalocyanine compounds, limited clinical application, etc., and achieves high molar absorption coefficient, good amphiphilicity, and improved The effect of transmittance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of the ortho-bridged azacrown ether modified phthalocyanine shown in formula (1) of the present invention comprises the following steps:
[0040] (1) Preparation of bisphthalonitrile with the structure shown in the following formula:
[0041] ,
[0042] Using triethylene glycol and 3-nitrophthalonitrile as reactants, dimethyl sulfoxide as solvent, in the presence of potassium carbonate and under the protection of nitrogen, stir and react at room temperature ~ 45 ° C for 36 ~ 96 hours, through thin layer chromatography Monitor, terminate reaction when 3-nitrophthalonitrile is consumed substantially completely, purify target product by solvent method and recrystallization method; In the above-mentioned reaction, the feed molar ratio of triethylene glycol and 3-nitrophthalonitrile is 1:1.8~2.2, the amount of solvent needs 1~2ml for every mmol reactant 3-nitrophthalonitrile, and the consumption of potassium carbonate needs 1.5~3mmol for every mmol ...
Embodiment 1
[0060] The preparation and physical and chemical properties of ortho-bridged azacrown ether modified zinc phthalocyanine with the structure shown in formula (1):
[0061]
[0062] Formula 1)
[0063] Among them, M is Zn 2+ .
[0064] (1) Preparation of bisphthalonitrile with the structure shown in the following formula:
[0065]
[0066] With triethylene glycol (30mmol) and 3-nitrophthalonitrile (54~66mmol, preferably 60mmol) as reactants, with anhydrous DMSO as solvent (30~60ml, preferably 30ml), in potassium carbonate (80~ 180mmol, preferably 100mmol, added in batches) in the presence of nitrogen protection, stirred at room temperature to 45°C (preferably room temperature) for 48 to 96 hours, and monitored the end of the reaction by thin-layer chromatography. The reaction mixture was suction-filtered with a sand core funnel, the filtrate was collected, the filtrate was added to 500ml of ice-water mixture, stirred, a large amount of precipitate was precipitated, allo...
Embodiment 2
[0073] Preparation and properties of ortho-bridged azacrown ether modified zinc phthalocyanine with the structure shown in formula (2):
[0074]
[0075] Formula (2)
[0076] Among them, M is Zn 2+ .
[0077] Add 1.0 mmol of bisphthalonitrile obtained in Example 1, 2~5 mmol of phthalonitrile (preferably 4mmol) to 10~30ml (preferably 20ml) of n-pentanol (or N,N-dimethylformamide or dimethylethanolamine, preferably n-pentanol), nitrogen, stirred and heated to complete dissolution, then add 1~5 mmol (preferably 4mmol) anhydrous zinc acetate and 0.4~0.8ml (preferably 0.6ml) DBU, 130~ Stir the reaction at 150°C for 4-24 hours (monitor the end point of the reaction by thin-layer chromatography). After the solvent was removed by vacuum rotary evaporation, the reaction product was dissolved with a small amount of DMF, added to ice water, and the blue precipitate was collected by filtration, allowed to stand at 5°C, filtered with suction, washed with water, and freeze-dried to ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com