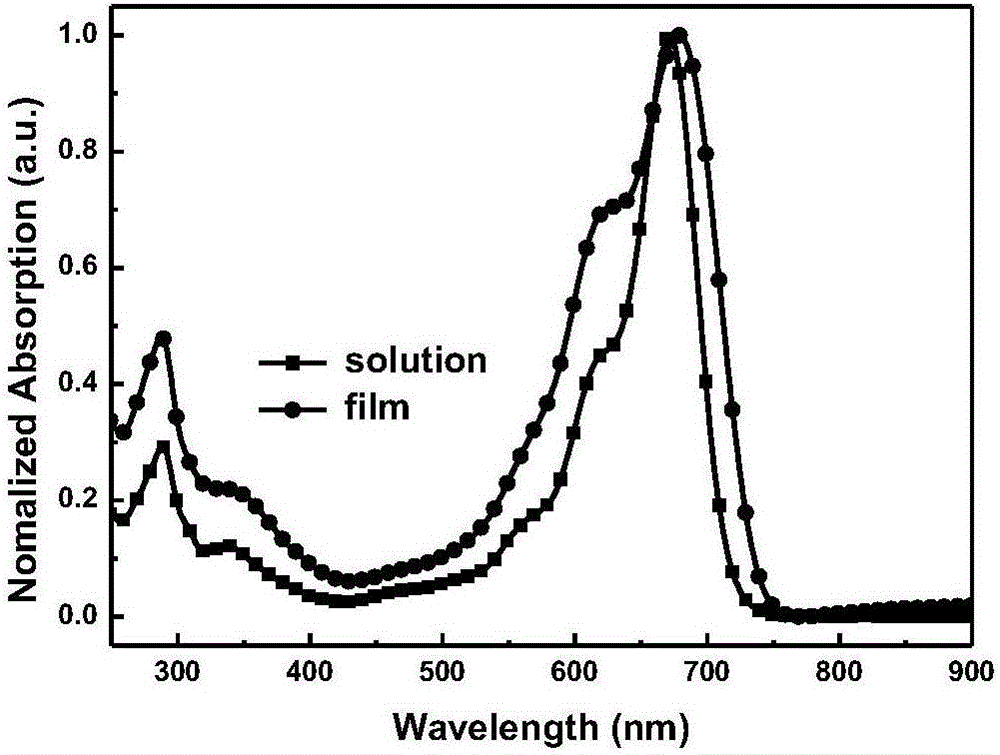
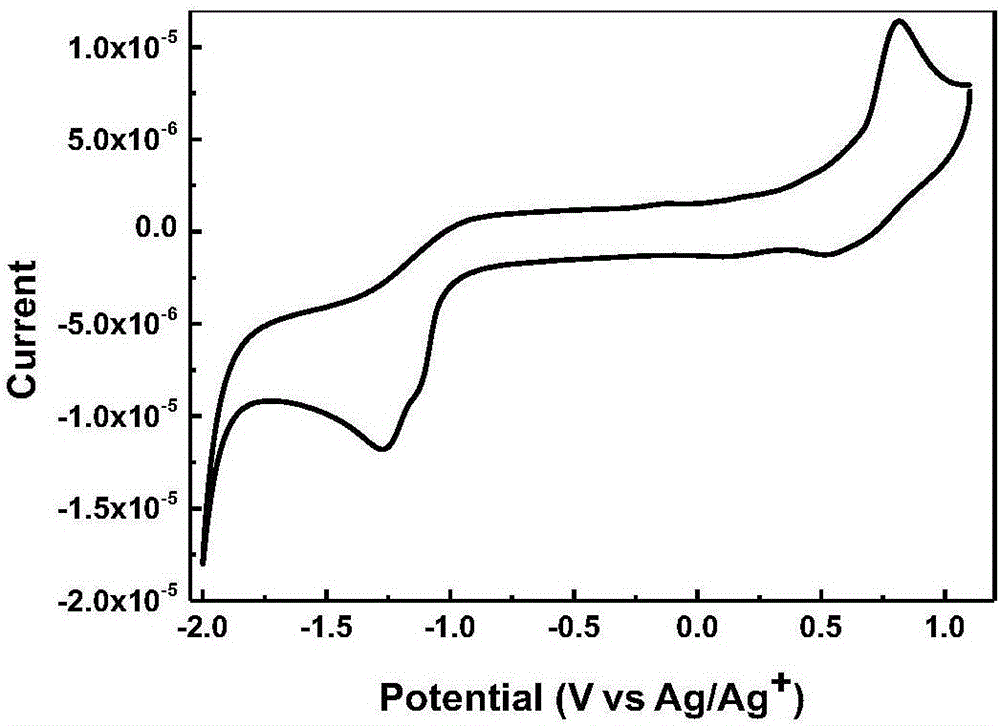
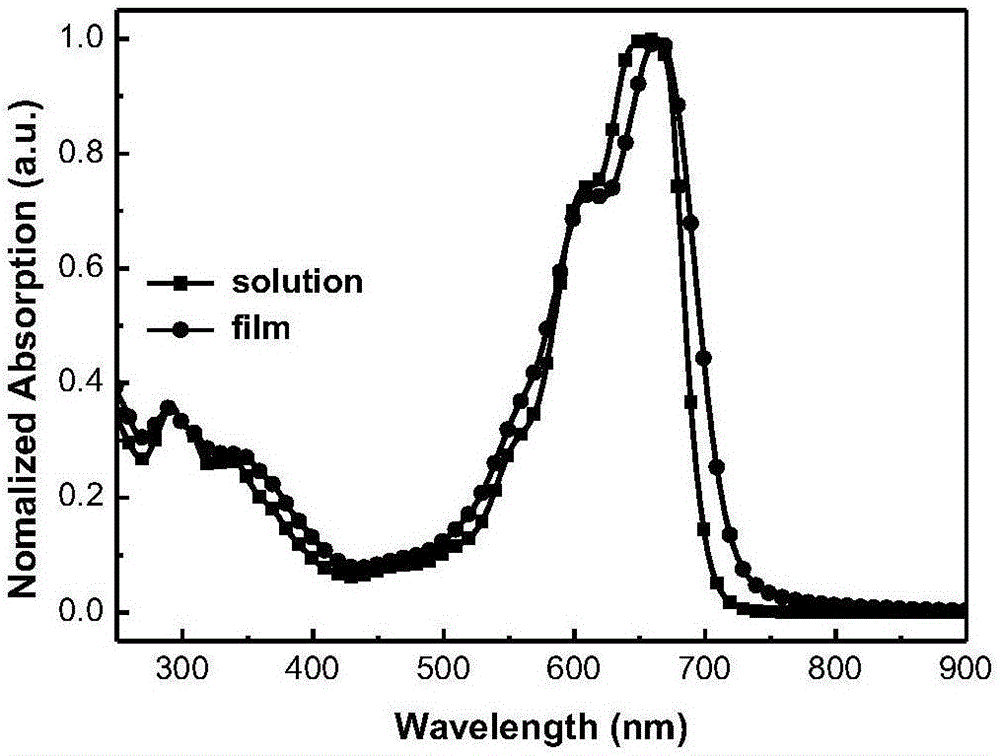
A-D-A type conjugated molecule based on dibenzo pentabasic fused heterocycle and preparation method of A-D-A type conjugated molecule
A technology of conjugated molecules, A-D-A, applied in electrical components, semiconductor/solid-state device manufacturing, photovoltaic power generation, etc., can solve problems such as difficult energy levels, weak absorption, and difficult purification, and achieve simple operation, strong absorption capacity, and good spectrum The effect of absorption range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthetic route of embodiment 1. compound 3a is as follows:
[0033]
[0034] Under nitrogen protection, carbazole borate compound 1a (6.57g, 10mmol), compound 2 (5.4g, 24mmol), potassium carbonate (8.2g, 60mmol), 336 (1g), Pd (PPh 3) 4 (1.15g, 10% mol) was dissolved in 90mL toluene / water (5:1), heated to reflux for 72h, stopped the reaction, extracted with ether, combined organic phases, washed with saturated brine, dried over anhydrous magnesium sulfate, suction filtered, spin The solvent was dried and separated by column chromatography to obtain 3.8 g of yellow oily substance, the yield was 56%. 1 H NMR (500MHz, CDCl 3 )δ8.15–8.05(m,2H),7.76(s,1H),7.55(d,J=5.4Hz,3H),7.36(m,2H),7.28(d,J=5.4Hz,2H), 4.55(tt,J=10.0,5.2Hz,1H),3.72(s,6H),2.28(m,2H),1.93(m,2H),1.27–1.09(m,24H),0.81(t,J= 7.1Hz,6H). 13 C NMR (125MHz, CDCl 3 )δ164.01, 152.23, 142.11, 130.16, 124.03, 120.90, 113.27, 110.45, 56.74, 51.55, 33.78, 31.75, 29.41, 29.34, 29.19, 26.94, 22.58, 14.04.
Embodiment 2
[0035] Embodiment 2. The synthetic route of compound 4a is as follows:
[0036]
[0037] Under the protection of nitrogen, 23.4 mL of 2M butyllithium was added dropwise to a 250 mL three-neck flask containing 4-hexylbromobenzene (11.3 g, 46 mmol) and 100 mL of THF at -78 °C, and the reaction was kept at low temperature for 1 h. At this temperature, compound 3a (3.567 g, 7.8 mmol) was added dropwise to the reaction liquid, returned to room temperature and stirred overnight, hydrolyzed, extracted three times with ether, combined organic phases, washed with saturated brine, and dried over anhydrous magnesium sulfate. After suction filtration, the solvent was spin-dried and separated by column chromatography to obtain 3.9 g of a yellow-green oil, with a yield of 60%. 1 HNMR (500MHz, CDCl 3 )δ7.86(s,1H),7.82(s,1H),7.52(s,1H),7.38(s,1H),7.27(d,J=4.8Hz,2H),7.18(d,J=7.8 Hz,8H),7.02(d,J=7.4Hz,10H),4.64–4.49(m,1H),2.56–2.50(m,8H),2.36–2.24(m,2H),2.03–1.92(m, 2H),1.65–1.49(m,16H),1...
Embodiment 3
[0038] The synthetic route of embodiment 3. compound 5a is as follows:
[0039]
[0040] Add compound 4a (3.9g, 1.2mmol) in a 50mL single-necked round bottom flask, dry CH 2 Cl 2 150mL, boron trifluoride ether solution 15mL, heated to reflux overnight. Turn off heat. Cool, add water, CH 2 Cl 2 Extracted three times, combined the organic phases, washed with saturated brine, and dried over anhydrous magnesium sulfate. After suction filtration, the solvent was removed by spin, and 3.2 g of yellow solid was obtained by column chromatography, with a yield of 83%. 1 H NMR (500MHz, CDCl 3 )δ7.86(s,1H),7.83(s,1H),7.52(s,1H),7.38(s,1H),7.27(d,J=4.9Hz,2H),7.18(d,J=8.3 Hz,8H),7.06–7.00(m,10H),4.63–4.49(m,1H),2.57–2.48(m,8H),2.41–2.23(m,2H),2.00-1.94(,2H),1.62 –1.47(m,16H),1.39–1.09(m,41H),0.86(t,J=6.9Hz,12H),0.79(t,J=6.9Hz,6H). 13 C NMR (125MHz, CDCl 3 )δ156.39,144.98,143.08,143.04,141.08,135.11,134.66,128.20,127.93,127.18,123.36,102.15,99.54,62.20,56.81,35.59,33.81,31.78,3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com