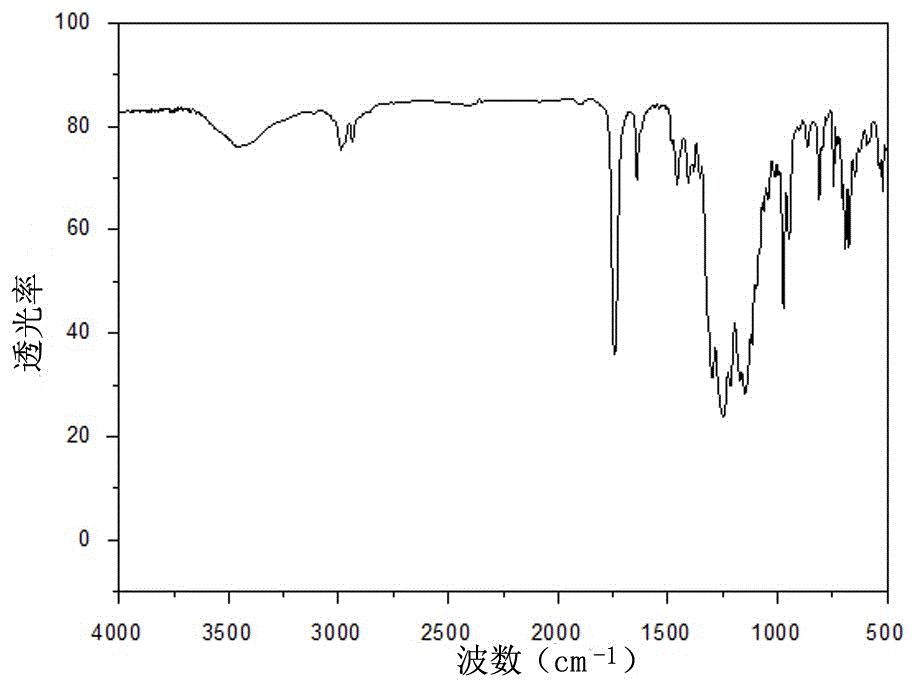
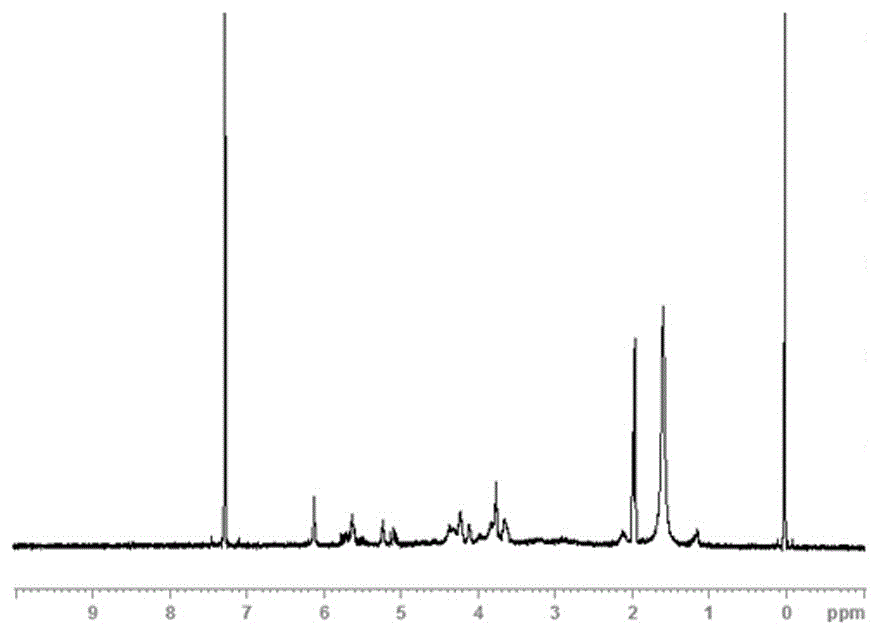
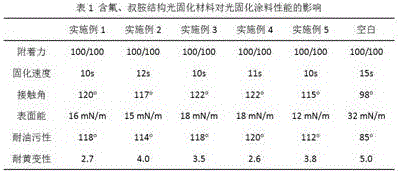
Photocuring material containing fluorine and tertiary amine structure and preparing method of photocuring material
A light-curing material and tertiary amine technology, which is applied in the field of fluorine-containing ultraviolet light-curing materials and their preparation, can solve the problems of complex process, difficulty, and low surface energy, and achieve simple synthesis process, avoid serious yellowing, and reduce oxygen resistance. The effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 500 mL four-necked flask equipped with a stirring device, a nitrogen device, and a condenser, add 211.0 g of dodecafluoroheptyl methacrylate, and drop 50.0 g of diethanolamine into the four-necked flask at 60°C, and react until the infrared The intermediate product A was obtained until the characteristic peak of acrylate was not detected.
[0024] In a 250 mL four-necked flask equipped with a stirring device, a nitrogen device, and a condenser, add 88.8 g of isophorone diisocyanate, raise the temperature to 65°C, and drop 46.4 g of hydroxyethyl acrylate into the four-necked flask within 5 hours After the dropwise addition, keep the temperature at 70°C until the isocyanate value is around 12.4%, and the intermediate product B is obtained.
[0025] Raise the temperature of the above-mentioned intermediate product B to 80°C, take 101.0 g of the intermediate product A and add it dropwise to B, and react until the characteristic peak of isocyanate group is not detected ...
Embodiment 2
[0031]In a 500 mL four-necked flask equipped with a stirring device, a nitrogen device, and a condenser, add 73.3 g of trifluoroethyl acrylate, and drop 50.0 g of diethanolamine into the four-necked flask at 60°C, and react until it cannot be detected by infrared The intermediate product A is obtained until the characteristic peak of acrylate.
[0032] In a 250 mL four-necked flask equipped with a stirring device, a nitrogen device, and a condenser tube, add 69.6 g of toluene diisocyanate, raise the temperature to 65°C, and drop 46.4 g of hydroxyethyl acrylate into the four-necked flask within 5 hours. After the addition is complete, keep warm at 70°C until the isocyanate value is around 14.4%, and the intermediate product B is obtained.
[0033] Raise the temperature of the above-mentioned intermediate product B to 80°C, take 51.8 g of intermediate product A and add it dropwise to B, and react until the characteristic peak of isocyanate group is not detected by the infrared s...
Embodiment 3
[0035] In a 500 mL four-necked flask equipped with a stirring device, a nitrogen device, and a condenser tube, add 83.8 g of trifluoroethyl methacrylate, and drop 50.0 g of diethanolamine into the four-necked flask at 60°C, and react until infrared detection The intermediate product A was obtained until the characteristic peak of acrylate was reached.
[0036] Add 105.0 g of dicyclohexylmethane diisocyanate to a 250 mL four-necked flask equipped with a stirring device, a nitrogen device, and a condenser tube, raise the temperature to 65°C, and add 54.8 g of hydroxyethyl methacrylate dropwise to the four-necked flask within 5 hours. In the mouth bottle, after the dropwise addition, keep it warm at 70°C until the isocyanic acid value is around 10.5%, and the intermediate product B is obtained.
[0037] Raise the temperature of the above-mentioned intermediate product B to 80°C, take 54.6 g of intermediate product A and add it dropwise to B, and react until the characteristic pea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com