A kind of * class derivative containing quinazoline group and application thereof
A quinazoline-based, quinazoline-containing technology, applied in the field of organic electroluminescence, can solve the problems of increasing the complexity of the device manufacturing process, reducing the cost of OLED, disadvantage, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
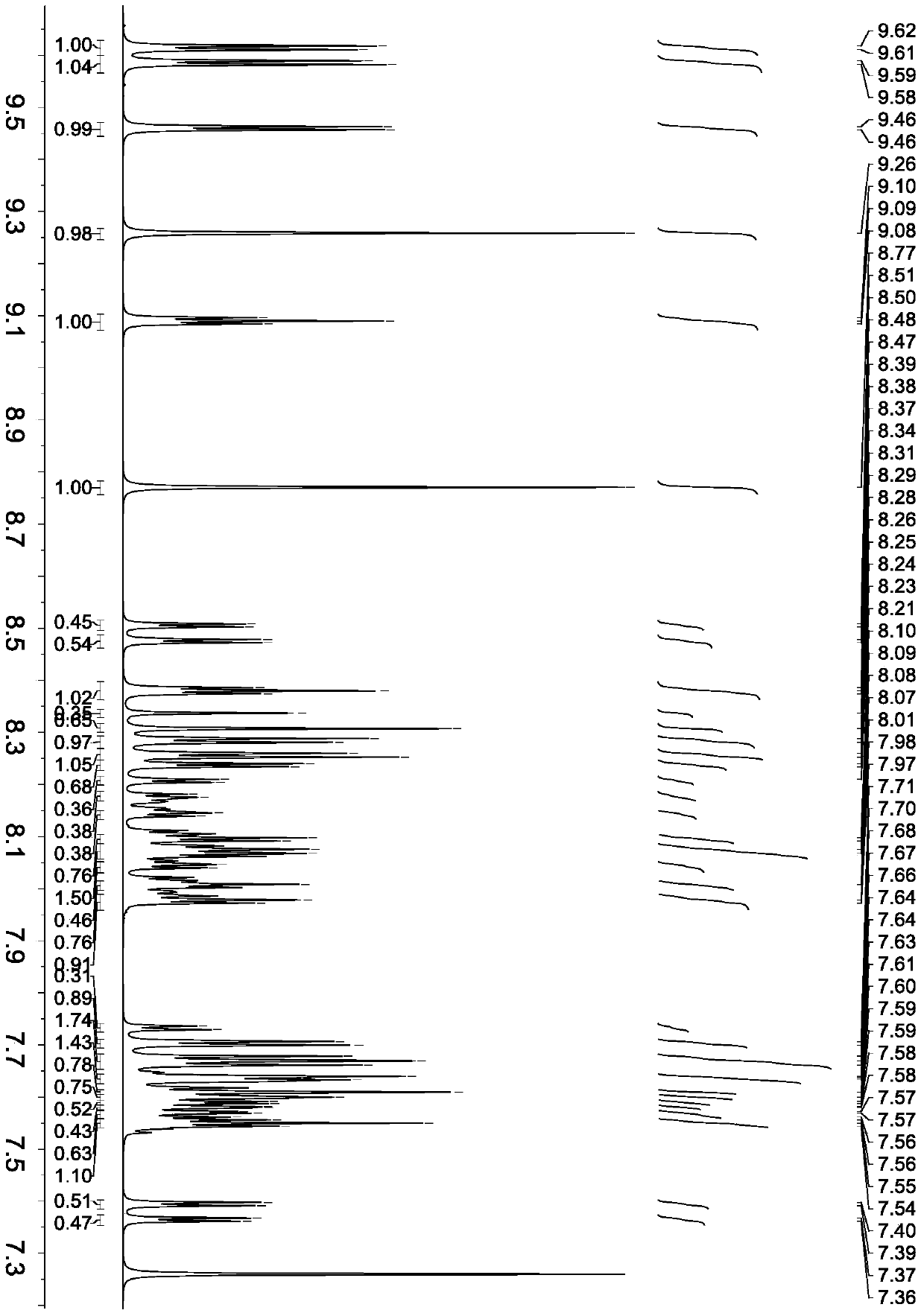
[0053] Example 1 Synthesis of various quinazoline derivative intermediates
[0054] Synthesis of 1,5-bromo-2-aminobenzylamine
[0055]
[0056] Add 5.9 g (molecular weight 196, 0.030 mol) of 2-amino-5-bromobenzonitrile to a 500 ml three-necked flask, and then add 45 ml of anhydrous tetrahydrofuran, stir to dissolve, and cool to 0°C. Under 0° C., nitrogen protection and stirring, 40 ml of borane tetrahydrofuran (concentration 1M) was slowly added to the three-necked flask. Stir at room temperature for three days. Cool to 0°C, add 20ml of absolute ethanol, and blow dry 1200ml of HCl gas. Then it was concentrated, cooled to 25°C, and 30 ml of isopropyl ether was added to precipitate the product. Then, under cooling and nitrogen protection, the solid product was added to 50 ml ammonia water, stirred, extracted with ethyl acetate, and the solvent was evaporated to obtain a solid product, which was dried to obtain 5.4 g, a molecular weight of 200, and a yield of 89.6%.
[0057] Synthes...
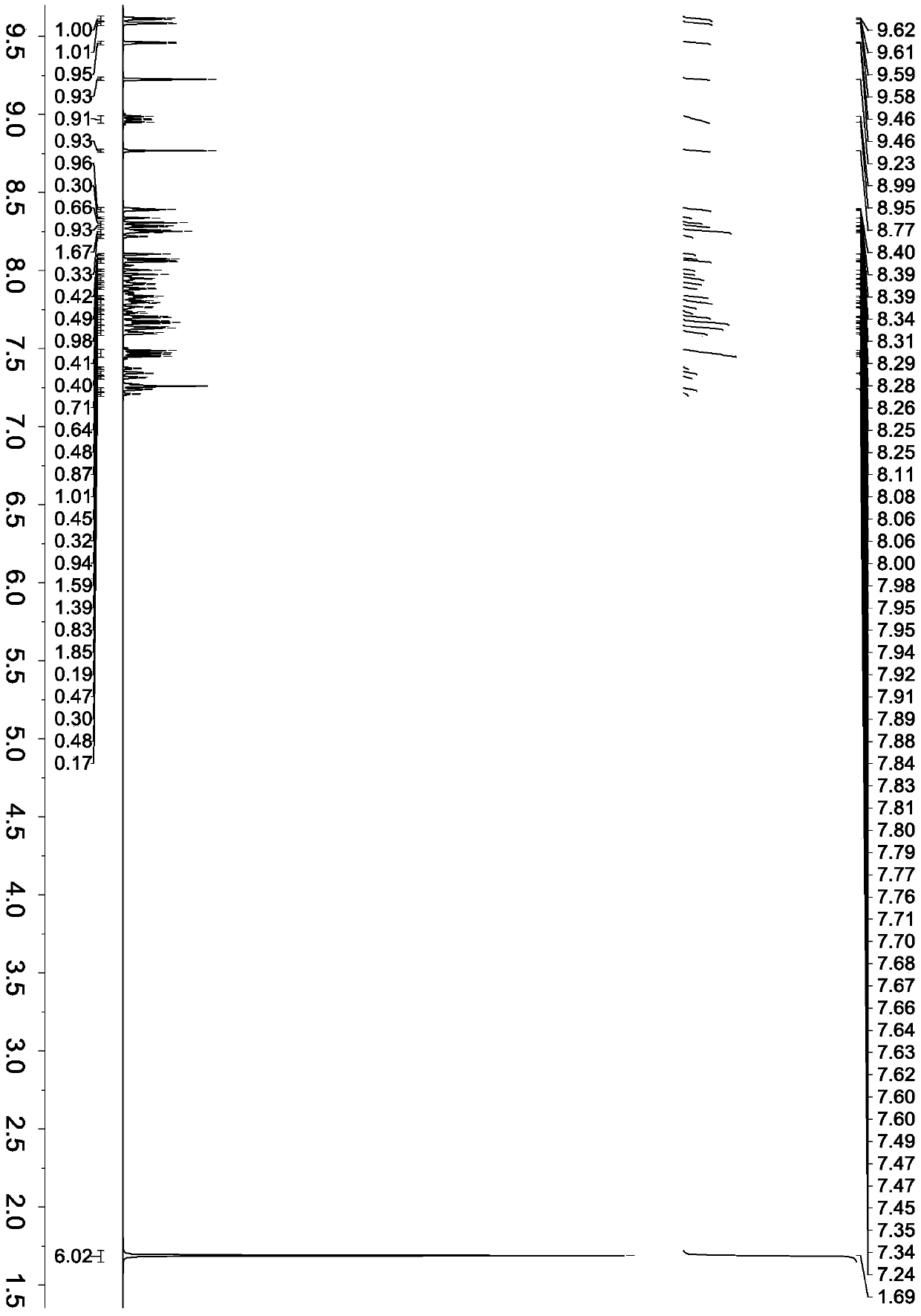
Embodiment 2
[0067] Synthesis of compound represented by formula (5)
[0068]
[0069] 1000ml bottle with magnetic stirring, add 6.68g 6-bromo-2-(naphthalene-2-yl)quinazoline (molecular weight 334, 0.02mol), 12-(naphthalene-2-yl) -6-Boric acid 8.76g (molecular weight 398, 0.022mol), tetrakis ((triphenylphosphine) palladium 1.16g (molecular weight 1154, 0.001mol), 2M sodium carbonate aqueous solution 80ml, toluene 80ml, ethanol 80ml. Replacement with argon After that, reflux and monitor the reaction by thin-layer chromatography (TLC). After 4 hours, TLC found that the bromide of the raw material had reacted completely with only product spots. Cool down, separate the organic layer, evaporate to dryness, and separate by column chromatography, ethyl acetate / petroleum ether After rinsing, 10.6 g of the compound represented by formula (5) with a molecular weight of 608 and a yield of 86.7% were obtained.
[0070] Product MS (m / e): 608, elemental analysis (C 46 H 28 N 2 ): Theoretical value C: 90.76%...
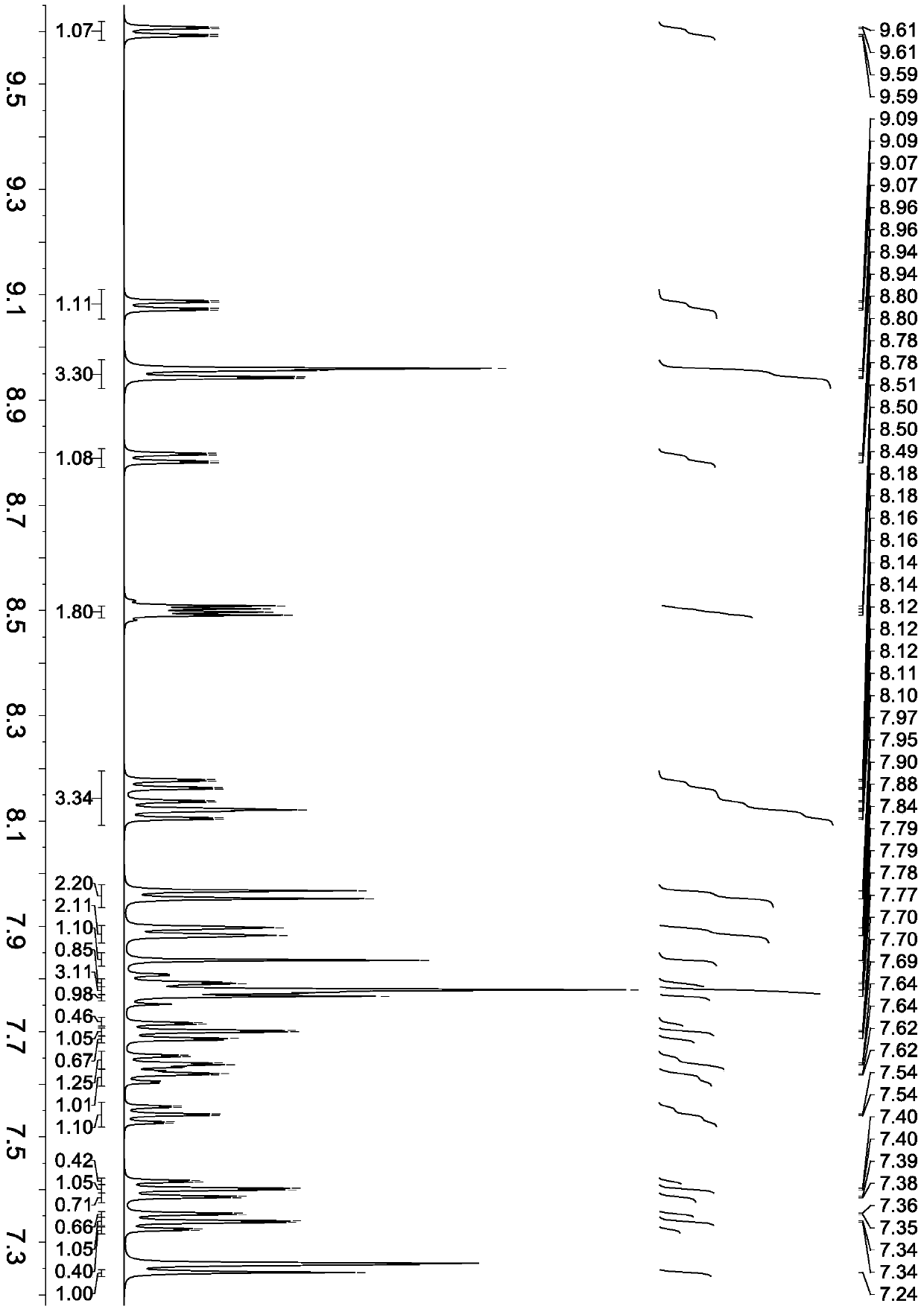
Embodiment 3
[0072] Synthesis of compound represented by formula (6)
[0073] The synthesis procedure is the same as in Example 2, except that 6-bromo-2-(naphthalene-2-yl)quinazoline is changed to 6-bromo-2-(naphthalene-1-yl)quinazoline, and other reagents remain unchanged. The compound represented by formula (6) is obtained.
[0074] Product MS (m / e): 608, elemental analysis (C 46 H 28 N 2 ): Theoretical value C: 90.76%, H: 4.64%, N: 4.60%; measured value C: 90.73%, H: 4.63%, N: 4.64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com