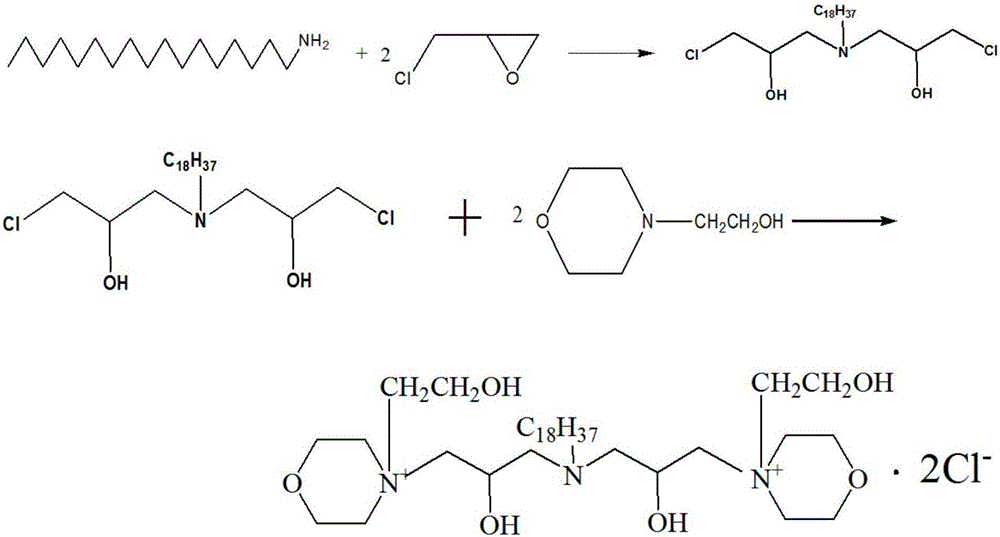
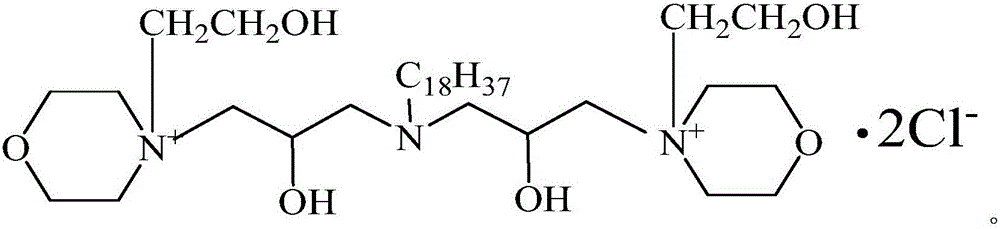
Bi(1-chlorine-N-hydroxyethyl morpholinium-3-hydroxy propyl group)n-octadecylamine quaternary ammonium salt and preparation method thereof
A technology of hydroxyethyl morpholinium and n-octadecylamine, which is applied in the field of di-n-octadecylamine quaternary ammonium salt and its preparation, can solve the problem of poor slow release performance, single synthetic substance performance, and poor water solubility of metronidazole and other problems, to achieve the effect of simplifying the post-reaction treatment, optimizing the reaction conditions, and improving the corrosion inhibition effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of two (1-chloro-N-hydroxyethylmorpholinium-2-hydroxypropyl) n-octadecylamine quaternary ammonium salt may further comprise the steps:
[0044] (1) First add 10.70g (0.05mol) of octadecylamine and 40mL of absolute ethanol to the three-necked flask, stir continuously at 300r / min, raise the temperature to 40°C, add 9.25g (0.1mol) of epichlorohydrin dropwise, and reflux the reaction 12h. Distilled under reduced pressure and washed with acetone to obtain bis(1-chloro-2-hydroxypropyl) n-octadecylamine as a viscous liquid intermediate.
[0045] (2) Add 18.5 g (0.05 mol) of the intermediate prepared above, 13.1 g (0.1 mol) of N-hydroxyethylmorpholine and 40 mL of absolute ethanol into another three-necked flask, and react under reflux at 80° C. for 3 h. Ethanol is distilled off, washed with isooctane, placed to precipitate crystals, washed with acetone and purified, and dried in vacuum to obtain bis(1-chloro-N-hydroxyethylmorpholinium-2-hydroxypropyl) n-octade...
Embodiment 2
[0047] The preparation of two (1-chloro-N-hydroxyethylmorpholinium-2-hydroxypropyl) n-octadecylamine quaternary ammonium salt may further comprise the steps:
[0048] (1) First add 3g of octadecylamine to the three-necked flask, then add 10ml of absolute ethanol to dissolve it, then heat up to 50°C, stir and add 2.07g of epichlorohydrin (the drop rate is 30 drops / min), Reflux reaction 8h. After the reaction, the ethanol was distilled off under reduced pressure, washed with acetone, and the acetone was removed by rotary evaporation to obtain bis(1-chloro-2-hydroxypropyl) n-octadecylamine, a viscous liquid intermediate.
[0049] (2) Add 2.5 g of the intermediate prepared in (1) to another three-necked flask, then add 1.31 g of N-hydroxyethylmorpholine and 4 ml of absolute ethanol, and heat up to 50° C. for 8 hours. Rotary evaporation to remove ethanol, wash with isooctane, stand to precipitate crystals, wash with acetone and purify, and dry in vacuum to obtain bis(1-chloro-N-hy...
Embodiment 3
[0051]The preparation of two (1-chloro-N-hydroxyethylmorpholinium-2-hydroxypropyl) n-octadecylamine quaternary ammonium salt may further comprise the steps:
[0052] (1) First add 3g of octadecylamine to the three-necked flask, then add 10ml of absolute ethanol to dissolve it, then heat up to 60°C, stir and add 2.07g of epichlorohydrin (the drop rate is 30 drops / min), Reflux reaction 8h. After the reaction, the ethanol was distilled off under reduced pressure, washed with acetone, and the acetone was removed by rotary evaporation to obtain bis(1-chloro-2-hydroxypropyl) n-octadecylamine, a viscous liquid intermediate.
[0053] (2) Add 2.5 g of the intermediate prepared in (1) to another three-necked flask, then add 1.31 g of N-hydroxyethylmorpholine and 4 ml of absolute ethanol, and heat up to 60° C. for 7 hours. Rotary evaporation to remove ethanol, wash with isooctane, stand to precipitate crystals, wash with acetone for purification, and vacuum dry to obtain bis(1-chloro-N-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com