2-thiophenecarboxaldehyde derivative industrial manufacture method
A technology of thiophene derivatives and thiophene formaldehyde, applied in the direction of organic chemistry, can solve the problems of complex by-products, difficult separation, environmental pollution, etc., and achieve the effects of convenient post-processing, simple production methods, and no three wastes pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
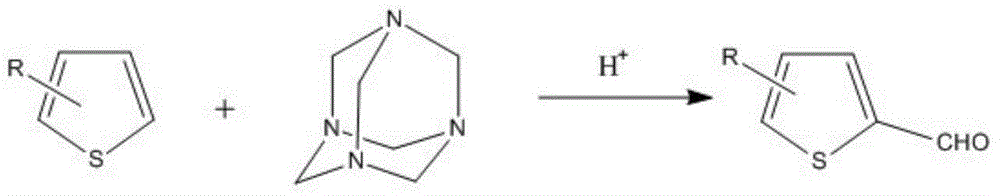
[0035] The reaction equation is as follows:
[0036]
[0037] Put 840g of thiophene and 1400g of hexamethylenetetramine into the reaction kettle, and add 35% phosphoric acid aqueous solution while stirring until the raw materials are dissolved. React at a temperature of 100° C. for 4 hours; distill under reduced pressure to obtain 1060 g of 2-thiophenecarbaldehyde. Or obtain compound B 1080g through rectification.
Embodiment 2
[0039] The reaction equation is as follows:
[0040]
[0041] Put 1230g of compound A and 2800g of hexamethylenetetramine into the reaction kettle, and add trifluoroacetic acid while stirring until the raw materials are dissolved. React at a temperature of 70-90°C for 3-6 hours; evaporate the solvent under reduced pressure, add a saturated aqueous solution of sodium carbonate to neutralize the system until the pH is 6-8, add 500ml of toluene solvent and water to extract twice, take the organic layer, The solvent was distilled off under reduced pressure to obtain 1460 g of compound B.
Embodiment 3
[0043] The reaction equation is as follows:
[0044]
[0045] Put 1200g of compound A and 2100g of hexamethylenetetramine into the reaction kettle, and add 50% formic acid aqueous solution while stirring until the raw materials are dissolved. React at a temperature of 80°C for 8 hours; evaporate the solvent under reduced pressure, add a saturated aqueous solution of sodium carbonate to neutralize the system until the pH is 6-8, add 500ml of ethyl acetate solvent and water to extract twice, take the organic layer, add sulfuric acid Sodium was absorbed into water, filtered, and the solvent was distilled off under reduced pressure to obtain 1501 g of compound B. Embodiment four,
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com