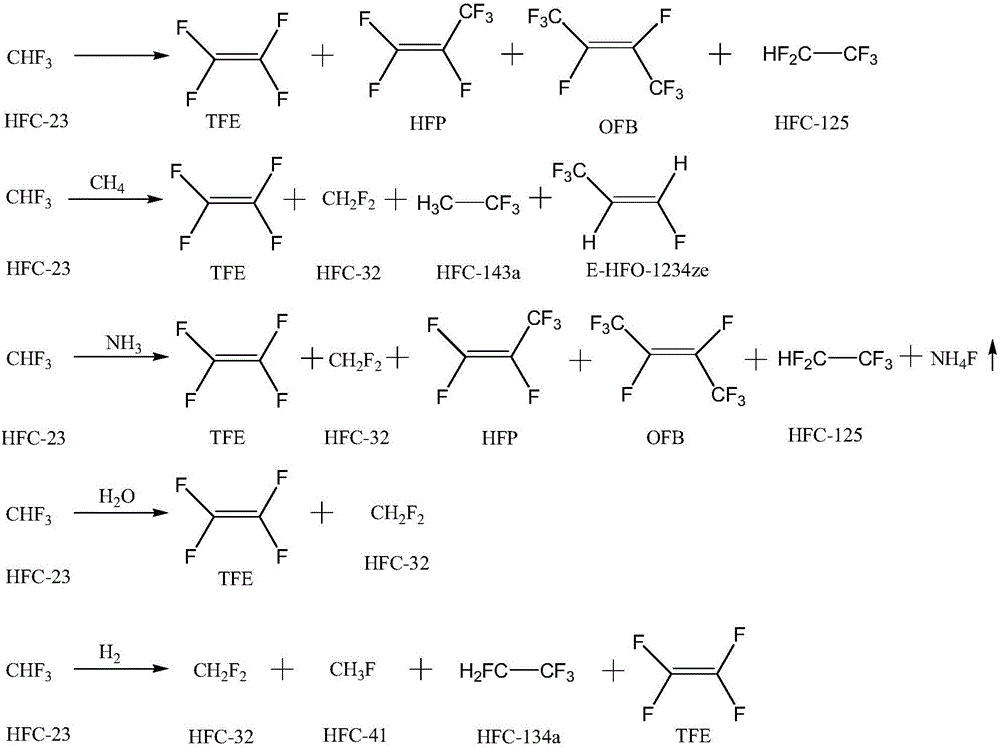
Method for preparing fluorinated compound CFR1=CFR2 (R1, R2=F or -CF3) through trifluoromethane thermolysis
A technology for trifluoromethane and compounds, which is applied in the field of preparing fluorine-containing compounds CFR1=CFR2 and pyrolysis of trifluoromethane alone, can solve the problems of easy collapse of the structure, being in a molten state, and the reaction cannot be carried out normally, and achieves good repeatability, The effect of reducing stress and difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
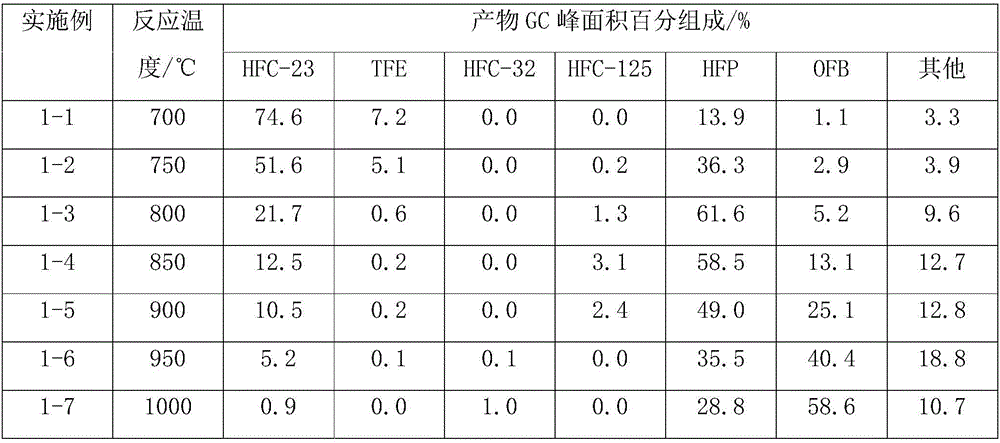
[0030] A tubular reactor made of Incon with an inner diameter of 1 / 2 inch and a length of 30 cm is heated to 700-1000 ° C, and trifluoromethane (HFC-23) is introduced for the reaction, and the residence time of trifluoromethane in the reactor is controlled to be After 15 seconds, the reaction pressure was normal pressure, the reaction product was washed with water, alkali washed, and dried to remove water, and after the reaction for 10 h, a gas phase sample was taken for gas chromatography detection, and the results are shown in Table 1.
[0031] Table 1 The reaction results of the pyrolysis of trifluoromethane alone
[0032]
Embodiment 2
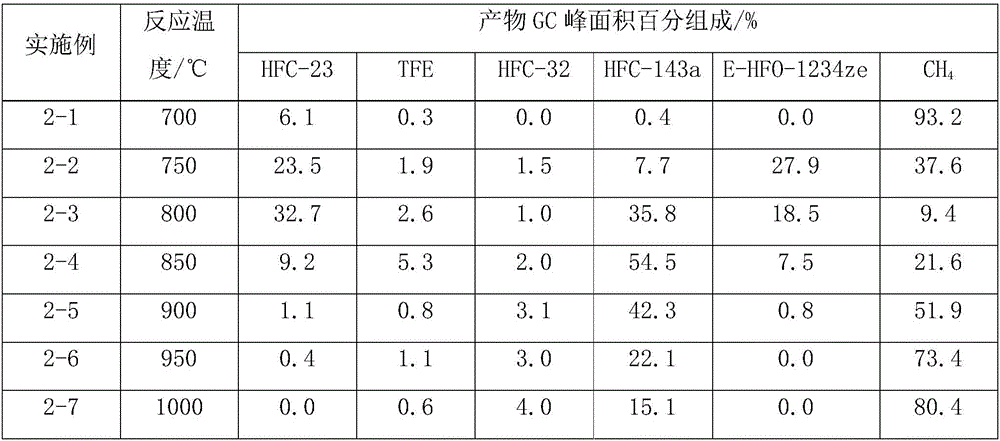
[0034] A tubular reactor made of Incon with an inner diameter of 1 / 2 inch and a length of 30 cm is heated to 700-1000 ° C, and trifluoromethane (HFC-23) is introduced to react with methane, and the molar ratio of trifluoromethane to methane is controlled to be 1:1, the residence time of trifluoromethane and methane in the reactor is 15 seconds, the reaction pressure is normal pressure, the reaction product is washed with water, alkali washed, dried to remove water, and after 10 hours of reaction, take a gas sample for gas chromatography detection , and the results are shown in Table 2.
[0035] Table 2 Reaction results of co-pyrolysis of trifluoromethane and methane
[0036]
Embodiment 3
[0038] A tubular reactor made of Incon with an inner diameter of 1 / 2 inch and a length of 30cm is heated to 700-1000°C, and trifluoromethane (HFC-23) is introduced to react with ammonia to control the moles of trifluoromethane and ammonia. The ratio is 1:1, the residence time of trifluoromethane and ammonia in the reactor is 15 seconds, and the reaction pressure is normal pressure. Gas chromatographic detection, the results are shown in Table 3.
[0039] Table 3 Reaction results of co-pyrolysis of trifluoromethane and ammonia
[0040]
[0041] *Other: Contains methane and other fluorinated compounds not listed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com