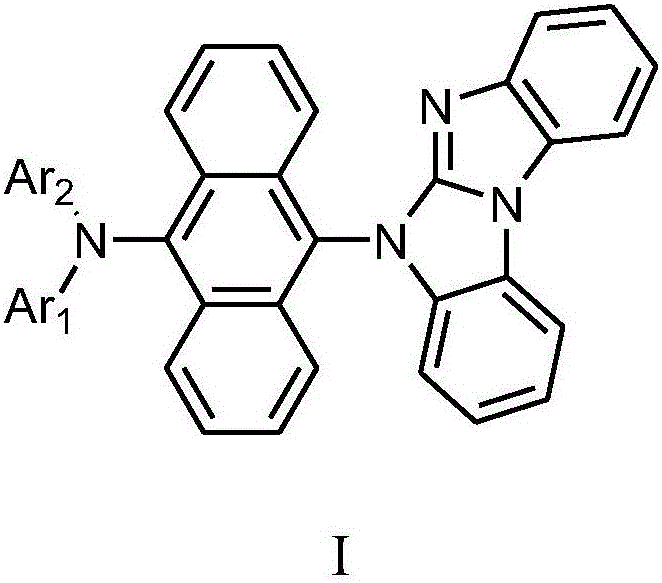
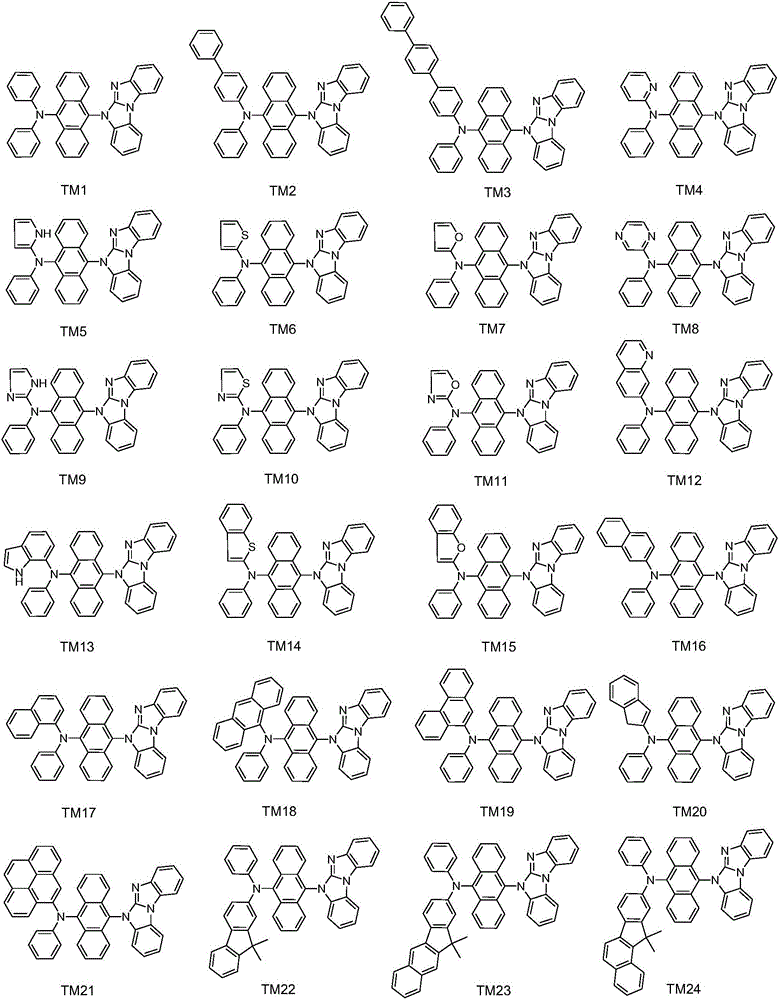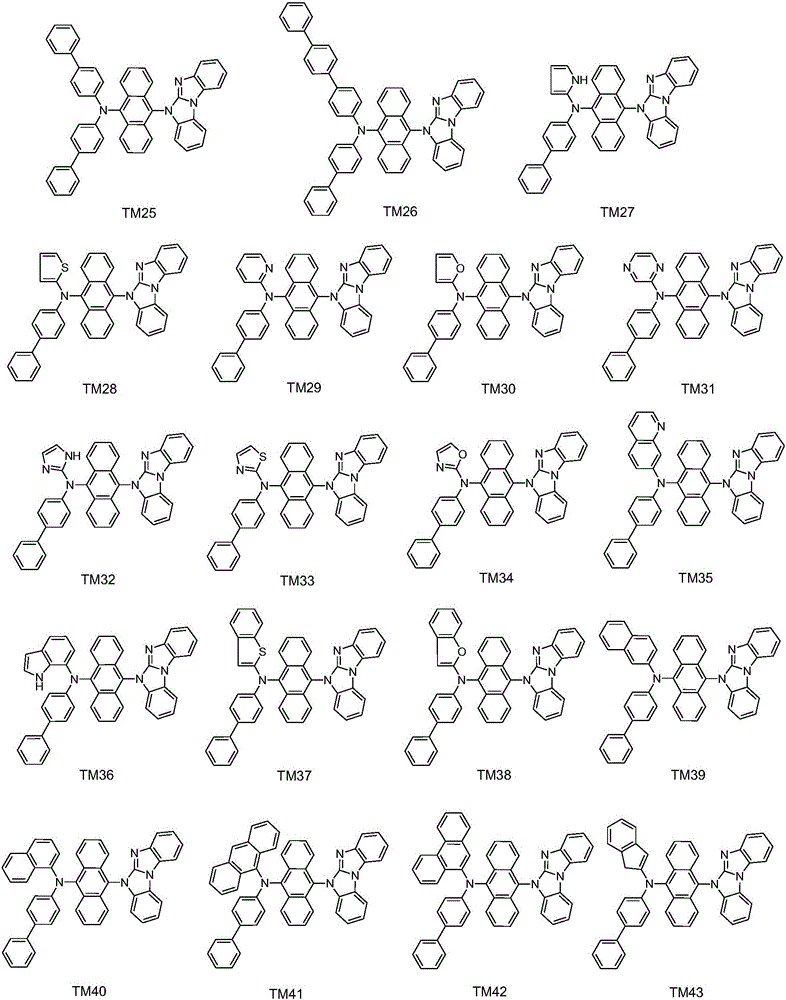Aromatic amine derivative, and preparation method and application thereof
A technology of aromatic amines and derivatives, which is applied in the field of aromatic amine derivatives and their preparation, can solve the problems of large volume of cathode ray tubes, poor temperature adaptability, and decreased response speed, and achieve good application effects and long-life crystallization , good film-forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0046] (A1) Preparation of intermediate C1
[0047]
[0048] To 9-aminoanthracene ( 35.3 g, 183 mmol) and bromobenzene (59.9 g, 384 mmol) in degassed toluene (500 mL), and the mixture was heated at reflux for 2 hours. The reaction mixture was cooled to room temperature, diluted with toluene and filtered through celite. The filtrate was diluted with water and extracted with toluene, and the combined organic phases were evaporated under vacuum. The residue was filtered through silica gel (heptane / dichloromethane) and crystallized from isopropanol. The resulting solid (46.2 g, 134 mmol) and NBS (28.6, 161 mmol) were dissolved in a mixed solution of DMF and chloroform (1:3, 60 mL), stirred at room temperature for 6 hours, added water and extracted with dichloromethane, and dried the organic phase. Concentration and crystallization from isopropanol gave intermediate C1 (45.3 g, 80% of theory).
[0049] Mass Spectrum m / z: 424.39 (calculated: 424.33). Theoretical element cont...
Embodiment A3
[0063] Example A3: Mass Spectrum m / z: 413.35 (calculated: 413.31). Theoretical element content (%)C 24 h 17 N 2 Br: C, 69.74; H, 4.15; N, 6.78; Br, 19.33 Measured element content (%): C, 69.78; H, 4.13; N, 6.78; Br, 19.35. The above results confirmed that the obtained product was the target product.
Embodiment A4
[0064] Example A4: Mass Spectrum m / z: 430.35 (calculated: 430.36). Theoretical element content (%)C 24 h 16 BrNS: C, 66.98; H, 3.75; Br, 18.57; N, 3.25; S, 7.45 Measured element content (%): C, 66.96; H, 3.73; Br, 18.51; The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com