Pure organic room-temperature phosphorescent material, preparation method thereof and application thereof
A room-temperature phosphorescent and optical material technology, applied in luminescent materials, organic chemistry, fluorescence/phosphorescence, etc., can solve the problems of heavy metals such as expensive, not environmentally friendly, and toxic, and achieve low toxicity, low cost, and easy synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The present application also provides a preparation method of the pure organic room temperature phosphorescent material, comprising the following steps:
[0055] With the compound of formula (Ⅲ) structure and NH 2 R is reacted in a solvent, and after separation, a pure organic room-temperature phosphorescent material having a structure of formula (I) is obtained;
[0056]
[0057] Wherein, R is a pure organic electron-donating group;
[0058] R 1 with R 2 Same, selected from pure organic electron-donating groups;
[0059] x 1 、X 2 、X 3 with X 4 Each is independently selected from H, Cl, Br or I.
[0060] The above preparation process prepares a pure organic room temperature phosphorescent material with the structure of formula (I) or formula (II), wherein when preparing the organic phosphorescent material with the structure of formula (II), the R 1 with R 2 Same, since the reactant with the structure of formula (Ⅲ) and NH 2 The molar ratio of R to the reac...
Embodiment 1
[0078] The synthesis of embodiment 1 naphthalimide derivative N1
[0079] In a 100ml round bottom flask, add 1,4,5,8-naphthalene tetracarboxylic anhydride (0.8g, 3mmol), p-methoxyaniline (0.37g, 3mmol) and 25ml absolute ethanol successively, and the mixture is under a nitrogen atmosphere Heating and reflux reaction for 2 hours, after the completion of the reaction, let it stand and cool to room temperature, filter the obtained solid and purify it by silica gel column chromatography, the eluent is ethyl acetate / n-hexane volume ratio 1 / 2, to obtain pure naphthalimide derivative N1 , light yellow powder, yield 92%. The hydrogen spectrum detection result is: 1 H NMR (DMSO-d 6 )δ (ppm): 3.85 (s, 3H), 7.10 (d, 2H, J = 8.8Hz), 7.36 (d, 2H, J = 8.8Hz), 8.62 (s, 2H), 8.74 (s, 2H) .
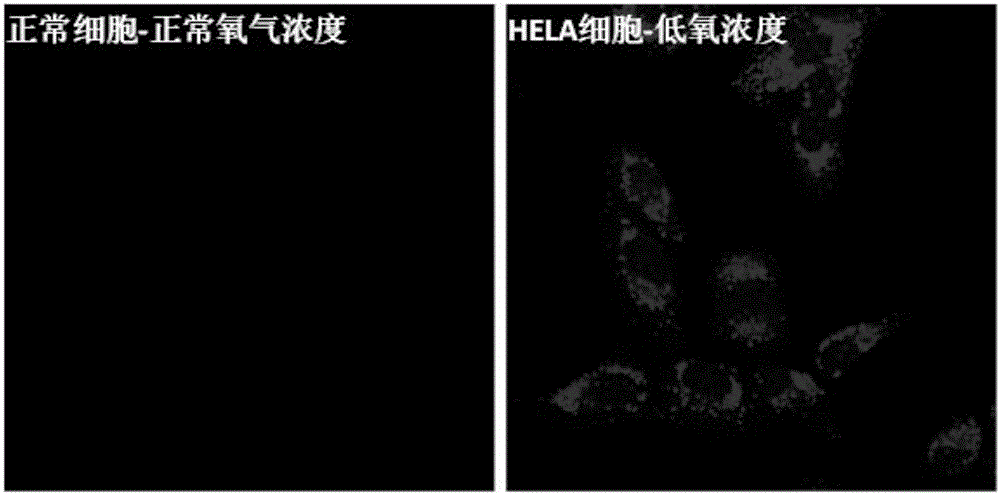
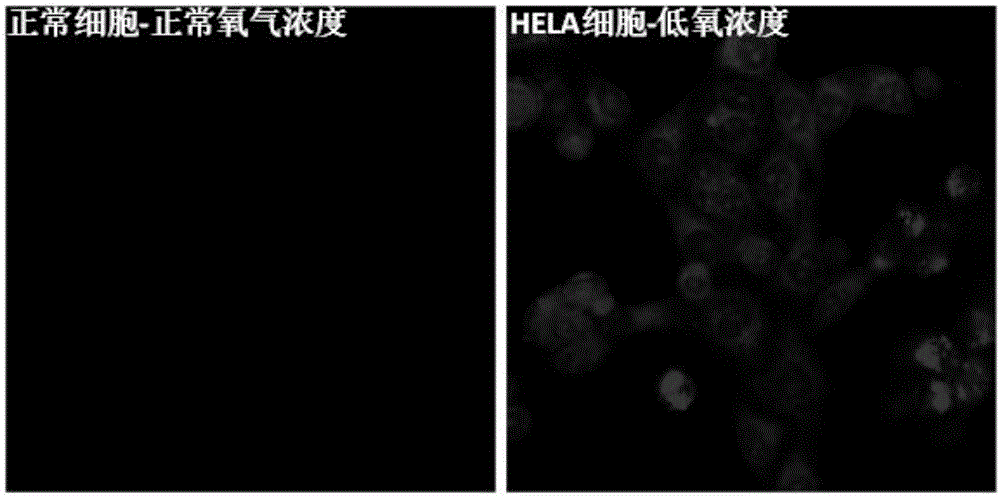
[0080] Mix N1 and PMMA at a mass ratio of 1 to 10,000 to form a film. In a pure nitrogen atmosphere, when irradiated by a 365nm ultraviolet lamp, there is red room temperature phosphorescence, such as ...
Embodiment 2
[0081] The synthesis of embodiment 2 naphthalimide derivatives N2
[0082] Replace the p-methoxyaniline in embodiment 1 with p-dimethylaminoaniline, and the others are the same as in embodiment 1. The result of the experiment is that a light yellow powder was obtained in this example, and the yield was 91% of the compound. The hydrogen spectrum detection result is: 1 H NMR (DMSO-d 6 )δ (ppm): 4.15 (s, 6H), 7.12 (d, 2H, J = 8.8Hz), 7.31 (d, 2H, J = 8.8Hz), 8.61 (s, 2H), 8.73 (s, 2H) .
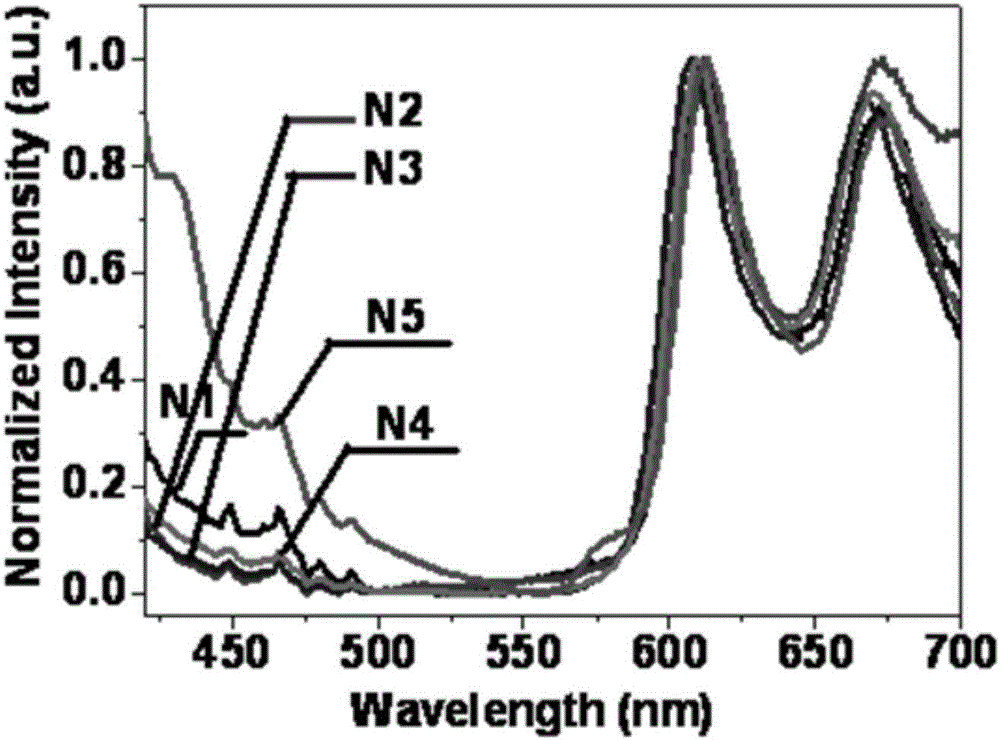
[0083] Mix N2 and PMMA at a mass ratio of 1 to 10,000 to form a film. In a pure nitrogen atmosphere, when irradiated by a 365nm ultraviolet lamp, there is red room temperature phosphorescence, such as figure 1 as shown, figure 1 is the photoluminescence spectrum of compounds N1-N5 under pure nitrogen atmosphere, from figure 1 It can be seen that the emission peaks are at 612nm and 671nm, the phosphorescence lifetime is 131ms, and the quantum yield is 5.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com