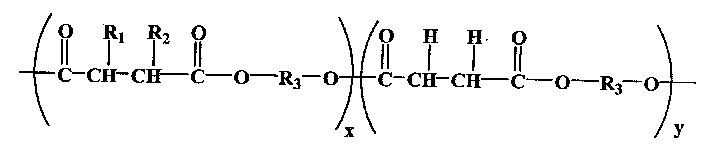Aliphatic polyester with hydrophilic functional lateral group and its prepn and use
A technology of aliphatic polyesters and side groups, applied in the field of aliphatic polyesters, can solve problems such as lack of active reaction sites, difficulty in adjusting hydrophilic/hydrophobic balance, and difficulty in polymer modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The structure and composition of the compound in the preparation method provided by the present invention are determined by solution nuclear magnetic resonance and infrared spectroscopy. The measurement temperature of nuclear magnetic resonance is room temperature, and the solvent is chloroform.
[0050] 【Purity and optical rotation of compound】
[0051] The purity and optical rotation of the compounds were determined by gas chromatography and optical rotation, respectively.
[0052] 【Thermophysical properties】
[0053] By differential scanning calorimetry (DSC), the melting point and glass transition temperature of the compound can be measured. And the thermal decomposition temperature of the compound can be measured by thermogravimetric analysis (TGA).
Embodiment 1
[0055] At room temperature, first add 10 g of benzyl alcohol and 100 ml of dichloromethane into a 250 ml flask and mix thoroughly, then add 100 ml of 50% aqueous potassium hydroxide and 15 mg of tetrabutylammonium bisulfate successively. After the above mixture was vigorously stirred at -15-10° C. for 5 minutes, 1.2 times the molar amount of trichloroacetonitrile was added dropwise. After the reaction system continued to stir for 30 minutes, it was heated to room temperature within 30 minutes. The oil and water phases in the reactant were separated, and the water phase was further extracted 2 times with dichloromethane. Then all the collected organic phase solutions were dried over anhydrous sodium sulfate and concentrated. The concentrated product was further purified on a silica gel column using n-hexane / dichloromethane mixed solution as mobile phase to obtain compound 8 benzyl trichloroacetimide. The final product Compound 8 was an oily substance with a yield of 95%. Com...
Embodiment 2
[0057] Add 30ml of cyclohexane / dichloromethane mixed solution with a volume ratio of 1:1 into a 100ml flask, then continue to add 15mmol of (s)-dimethylmalate and 18mmol of Compound 8 benzyl trichloroacetimide, followed by the slow dropwise addition of 0.2 ml of trifluoromethanesulfonic acid over 5 minutes. After the dropwise addition was completed, the temperature of the system was increased to 38° C. for reaction, and the reaction was carried out until the starting material detected by thin-layer liquid chromatography (TLC) was completely converted. At this point the reaction was quenched by adding 5 mL of saturated aqueous sodium bicarbonate and 100 mL of water. The white solid by-product trichloroacetimide in the reaction system will be removed by filtration, and at the same time, the oil and water in the filtrate will be separated, and the water phase will be extracted with ethyl acetate. The organic phase collected from the two phases was first dried with anhydrous sodi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com