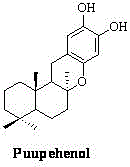
Synthetic method of marine natural product Puupehenol
A technology of natural products and synthetic methods, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of low yield, long synthetic route, poor stereoselectivity, etc., and achieve good product selectivity, high total yield, and reaction The effect of fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
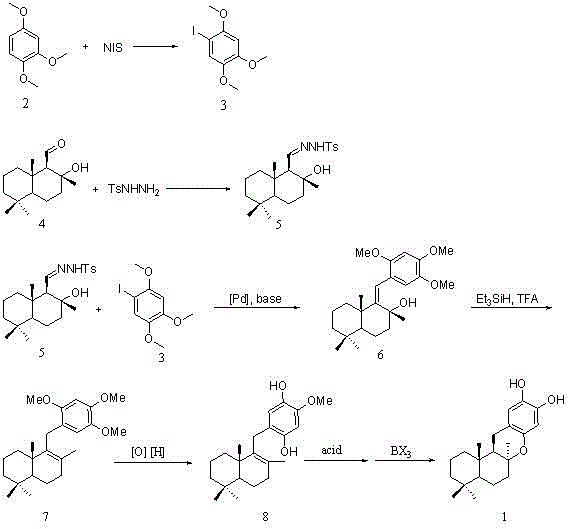
[0021] Example 1: Iodo 1,2,4-trimethoxybenzene (3, see figure 1 )Synthesis
[0022] Take 1.68 g (2, 10.0 mmol) of 1,2,4-trimethoxybenzene and dissolve it in 25 ml of anhydrous acetonitrile, add NIS (10.0 mmol), stir and react at room temperature for 2 hours, and TLC detects that the reaction is complete. Add 30 ml of water, extract with ethyl acetate (30mL x 3), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, and purify by column chromatography to give white solid iodo 1,2,4- Trimethoxybenzene 2.90 grams, yield is 99%.
Embodiment 2
[0023] Embodiment 2: (+) clary perilla hydrazone (5, see figure 1 )Synthesis
[0024] Take 2.38 g (+) sclarealdehyde (4, 10.0 mmol) and dissolve it in 30 ml of anhydrous methanol, add 1.86 g (10.0 mmol) of p-toluenesulfonyl hydrazide, stir and react at room temperature for 3 hours, and TLC detects that the reaction is complete. Concentrate to obtain white solid (+) sulfonyl hydrazone.
Embodiment 3
[0025] Embodiment 3: the basic framework of Puupehenol (6, see figure 1 )Synthesis
[0026] Take (+) 407 mg (5, 1.0 mmol) of clarylar sulfonylhydrazone and dissolve it in 4 ml of anhydrous tetrahydrofuran, add 23 mg (0.02 mmol) of tetrakistriphenylphosphine palladium and 622 mg (4.5 mmol) of potassium carbonate repeatedly Inflate and exhaust argon three times to exhaust the air. Another 294 mg iodo-1,2,4-trimethoxybenzene (3, see figure 1 ) was dissolved in 2 ml of anhydrous tetrahydrofuran, slowly added dropwise to the above reaction system, and the temperature was raised to 110 o C, the reaction was stirred for 10 hours, and the reaction was detected by TLC. Add 30 ml of water to the reaction system, extract with ethyl acetate (10 mL x 3), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, and purify by column chromatography to give a light yellow oil 315 mg, the yield was 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com