Hybrid antibacterial peptide based on fv7 anti-biofilm and its preparation method and application
A hybrid antimicrobial peptide and anti-biofilm technology, applied in the field of agricultural, animal husbandry and veterinary medicine, can solve the problems of less application in the field of medicine and animal husbandry, low antibacterial activity, etc., and achieve the goal of improving cell selectivity, enhancing antibacterial activity, and reducing costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Design and synthesis of antimicrobial peptides
[0016] LL: The 17th-29th amino acid functional sequence of the human antimicrobial peptide LL-37, this fragment is a peptide chain with an amphipathic helical structure rich in cations. The shortest sequence identified as having antibacterial and anticancer activities can effectively bind to anionic phospholipids of bacterial cell membranes and exhibit selective cytotoxicity, but no toxicity to human cells.
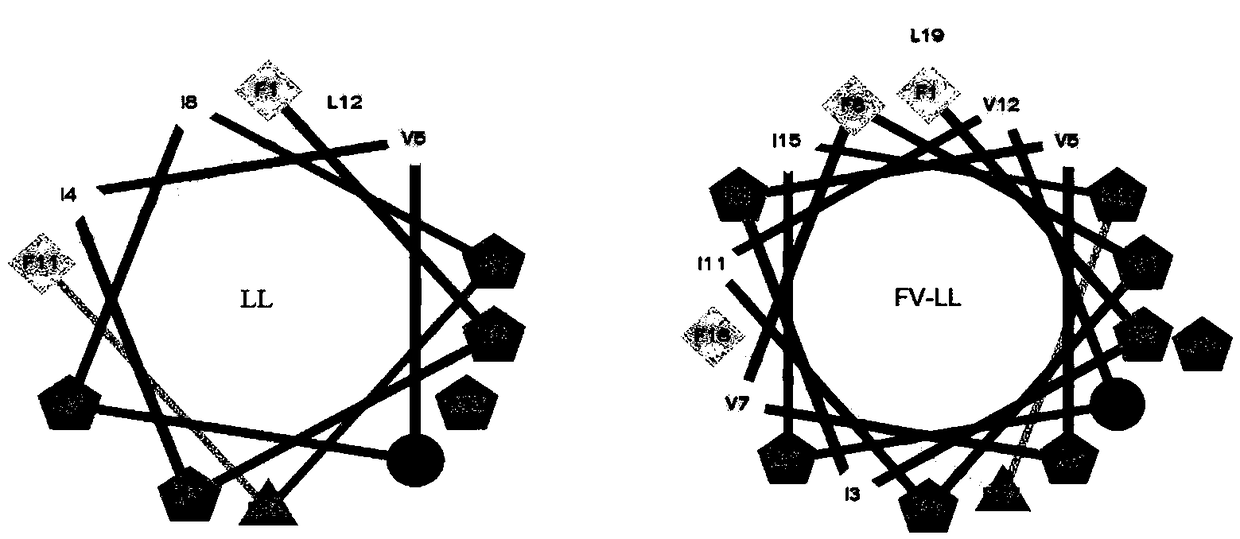
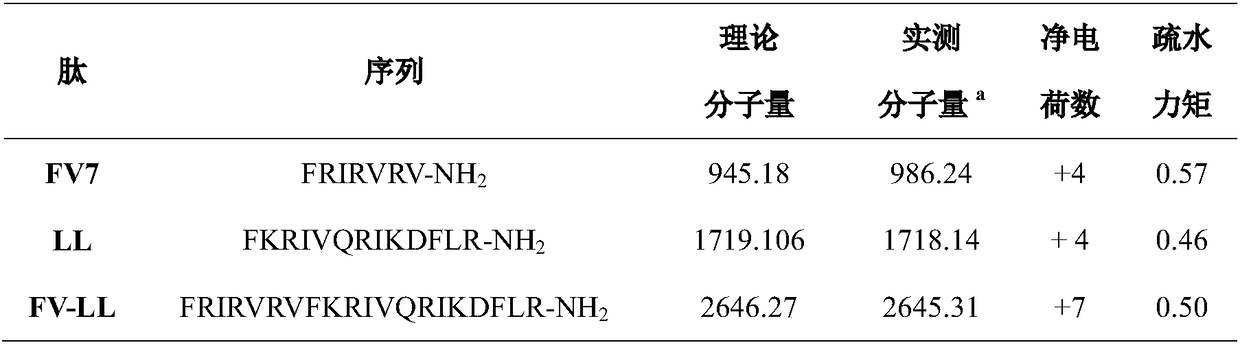
[0017] A sequence FV7 with anti-biofilm activity and a polypeptide fragment LL are hybridized to obtain a hybrid antibacterial peptide FV-LL. The helical wheel prediction diagram of the hybrid peptide FV-LL is as follows figure 1 shown. Using a peptide synthesizer, the above three antimicrobial peptides were synthesized by solid-phase synthesis. The sequences and physicochemical parameters of the three peptides are shown in Table 1.
[0018] Table 1 The sequences and physicochemical parameters of the th...
Embodiment 2
[0021] The physical and chemical index analysis of embodiment 2 antimicrobial peptides
[0022] The secondary structure of the antimicrobial peptide was analyzed by CD spectrum, and the results showed that both the hybrid peptide and the original peptide showed a random coil structure in the water environment, while in the hydrophobic environment (50% TFE) of the simulated microbial membrane and negative In the charged prokaryotic membrane environment (30mM SDS), both the original peptide LL and the hybrid peptide FV-LL showed a certain α-helical structure, while FV7 showed a certain β-sheet conformation.
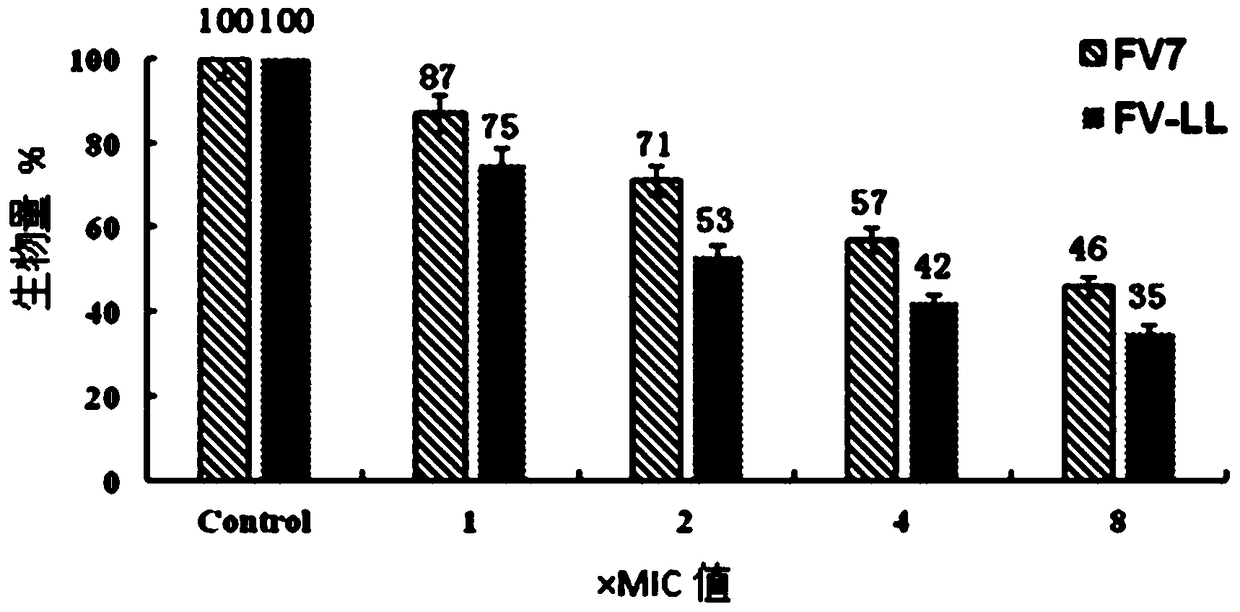
[0023] The stability analysis of antimicrobial peptides showed that the hybrid peptide FV-LL had ideal resistance to heat treatment. However, the antibacterial activity of the peptide FV-LL decreased slightly at different physiological concentrations of salt ions.
Embodiment 3
[0024] Bacteriostasis and anti-biofilm activity of embodiment 3 antimicrobial peptides
[0025] 1 Antibacterial activity
[0026] The peptide was configured as a 2.56mM / L storage solution for future use. The minimum inhibitory concentrations of several antimicrobial peptides were determined by the broth microdilution method. Using 0.01% acetic acid (containing 0.2% BSA) as the diluent, a series of gradient antimicrobial peptide solutions were sequentially prepared using the double dilution method. Take 100 μL of the above solution and place it in a 96-well cell culture plate, then add an equal volume of the bacteria solution to be tested (~10 5 individual / mL) in each well. Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor peptides) were set up. Incubate at a constant temperature of 37°C for 20 hours, and the minimum inhibitory concentration is the one where no turbidity is seen at the bottom of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com