Selenium modification method for improving immunological competence of radix pseudostellariae polysaccharides
The technology of immunological activity and modification method is applied in the field of selenization modification for improving the immunological activity of Polysaccharide Polysaccharide, which can solve the problems of barium chloride residue, polysaccharide efflux, low extraction rate of selenized polysaccharide, etc., so as to improve the yield and ensure the purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A selenization modification method for improving the immune activity of polysaccharides from Radix Pseudostellariae, said method comprising the following steps:
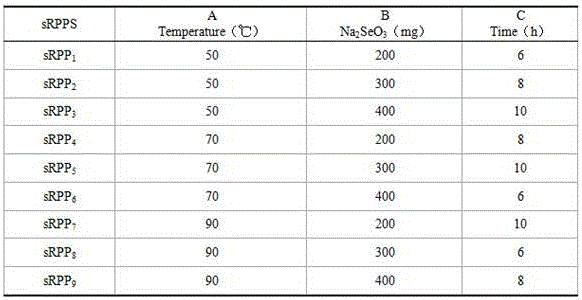
[0019] (1) Take 9 parts of 500 mg polysaccharides of Pseudostellaria Radix Pseudostellariae, put them in 50 mL of water, dissolve them under magnetic stirring, put them into three-neck flasks respectively, slowly add 2 mL of glacial acetic acid and 0.6 g of magnesium ferrite dropwise; add Na 2 SeO 3 200-400mg, react at 50-90℃ for 6-10h;
[0020] (2) Cool to room temperature after the reaction, adjust the pH value to 6 with anhydrous sodium carbonate, centrifuge, filter, and put the solution into a 1000 D dialysis bag for dialysis; take a sample of the dialysate every 6 hours, and use the ascorbic acid method to detect whether it contains sub- Sodium selenate, stop dialysis when there is no sodium selenite residue or no red color;
[0021] (3) The solution was subjected to Sephadex G-100 column chromatography...
Embodiment 2
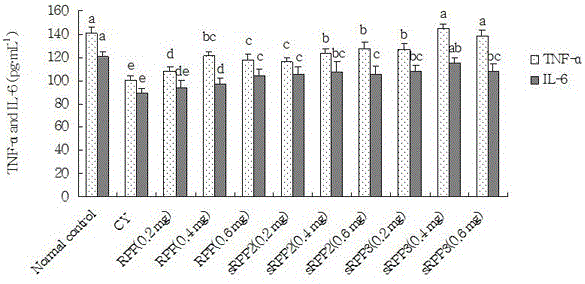
[0025] The mice had free access to food and water, the temperature was controlled at 22±1°C, and the light was cycled for 12 h. Mice were randomly divided into 11 groups, including healthy control group, drug treatment group, and cyclophosphamide (CY) model group, 20 in each group, and all animals were adaptively fed for 7 days. The healthy control group and the cyclophosphamide (CY) model group were subcutaneously injected with normal saline for 14 days. Administration group sRPP 2 , sRPP 3 , RPP was divided into three groups of three concentrations (1 mg / mL, 2 mg / mL, 3 mg / mL), a total of 9 groups, subcutaneous injection of 0.2 mL, continuous administration for 14 days. From the 15th day, except the healthy control group, 9 administration groups and cyclophosphamide (CY) model group received intraperitoneal injection of cyclophosphamide, 80 mg / kg·d, once a day, for 3 consecutive days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com