Isolation and in vitro expansion method of human vein endothelial cells
An endothelial cell and vein technology, applied in the field of bioengineering, can solve the problems of low cell positive rate, high cost, inconvenient operation, etc., and achieve the effects of broad application prospects, short time-consuming and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1, separation of human vein endothelial cells
[0061] This embodiment uses veins from human umbilical cord to realize the separation of human vein endothelial cells, including the following specific implementation process. It can be understood that the following operations are for the purpose of clearly disclosing the continuous process and not limiting the present invention:
[0062] 1) Take the fresh umbilical cord of a normal delivery baby in a sterile biological safety cabinet (within 12 hours, if the time is too long, the endothelial cells will die due to ex vivo, and it is difficult to survive), wash the umbilical cord twice with 250mL normal saline, and squeeze the umbilical cord with tweezers Tissue, remove the residual blood in the umbilical cord, and then use tweezers and a scalpel to peel off the umbilical cord amniotic membrane and jelly, and pick about 10cm of the human umbilical cord vein;
[0063] 2) Clamp both ends of the human umbilical cord...
Embodiment 2
[0067] Example 2, In Vitro Expansion of Human Vein Endothelial Cells
[0068] In this example, human vein endothelial cells were expanded in vitro, including the following specific operations: using platelet lysate (purchased from UltraGRO) containing 10% (5-15%, V / V), 10 ng / mL (8- VEGF (endothelial cell growth factor, purchased from peprotech), 2 units of heparin sodium (purchased from Wanbang Biochemical Medicine) and IMDM medium (purchased from gibco) (or DMEM / F12, α-MEM and other Cell culture medium) to resuspend human vein endothelial cells in expansion medium, at 37°C, 5% CO 2 , cultured under saturated humidity, induce adherence for 24 hours (12-36 hours are acceptable), change the medium in full, remove non-adherent cells, add fresh expansion medium, change the medium once every other day, and wait until the degree of cell fusion reaches When 90% (80-90% is acceptable), digest and passage with 0.05% (0.05-1% acceptable, M / V) trypsin (purchased from sigma) to obtain hu...
Embodiment 3
[0070] Embodiment 3, identification of human vein endothelial cells
[0071] 1. Growth characteristics and morphological characteristics of human vein endothelial cells
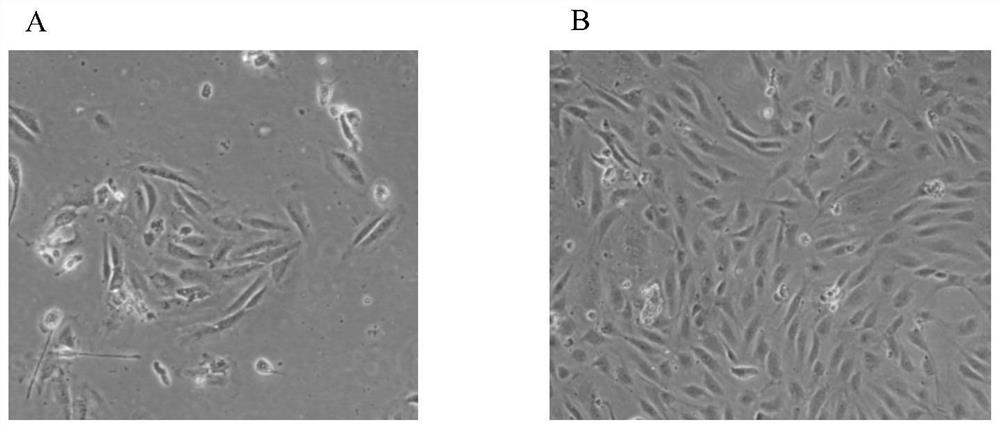
[0072] Observe the human vein endothelial cells (the human vein endothelial cells isolated in Example 1 are expanded) induced in Example 2 to adhere to the wall for 2 days with a microscope, as figure 1 As shown, there are scattered adherent cells in the shape of paving stones under the microscope. After the full amount of medium is changed and cultured for 3-4 days, the formation of monoclonal cells can be seen ( figure 1 A), when the cells are cultured until about 90% of the cell confluence ( figure 1 B), the cells are in the shape of typical paving stones, with fast proliferation and relatively uniform morphology, which can be used for the identification of cell biological characteristics.
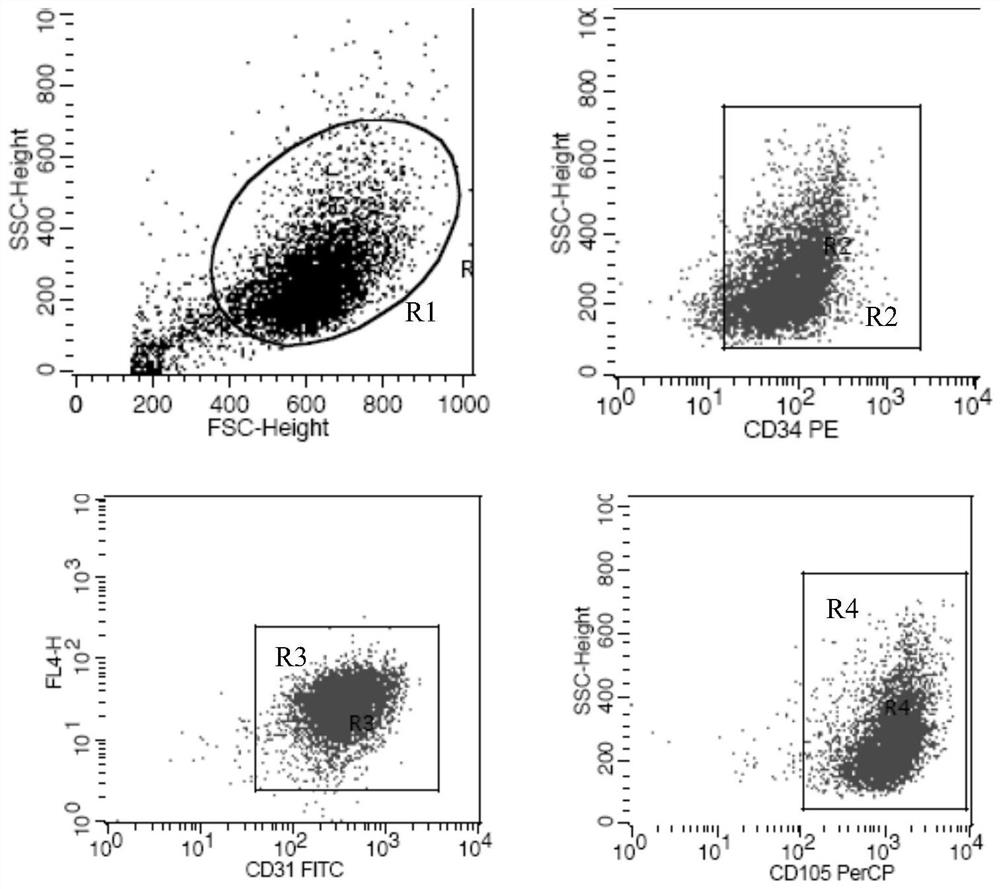
[0073] 2. Identification of surface markers of human vein endothelial cells by flow cytometry
[0074]The third g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com