Catalyst for production of methanol by CO2 hydrogenation
A catalyst and methanol production technology, which is applied in organic chemistry, bulk chemical production, hydroxyl compound preparation, etc., can solve the problems of low methanol selectivity, poor anti-poisoning performance, cumbersome preparation steps, etc., and achieve simple preparation process and high thermal conductivity The effect of inhibiting and preparing raw materials is cheap and easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
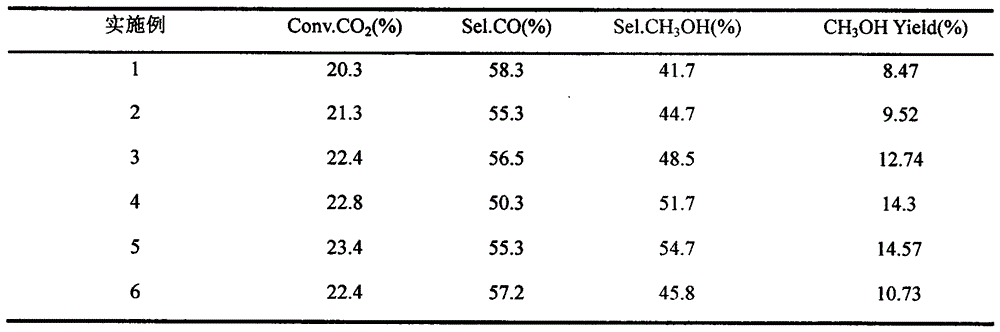
Examples
Embodiment 1
[0027] According to Cu / ZnO / ZrO 2 =4 / 3 / 3 mass ratio weighs 6.1gCu(NO 3 ) 2 ·3H 2 O, 5.5gZn(NO 3 ) 2 ·6H 2 O, 3.3gZrO(NO 3 ) 2 2H 2 O, dissolved in deionized water as solution A, weighed 7.1g of anhydrous NaCO by 1mol / L solution 3 , dissolved in deionized water as Solution B. Use a peristaltic pump to drop solution A and solution B at the same time, and the dropping rate is 150mL h -1 , the stirring speed is 900r / min, the reaction temperature is 70°C water bath temperature, and the pH is controlled to be 7. After the dropwise addition, continue to stir for 120min, then age at 30°C for 12h, filter, dry overnight at 90°C, and pass through N at 350°C. 2 Calcined for 5 hours, granulated with 20-40 meshes to obtain catalyst sample 1, recorded as CuO-ZnO-ZrO 2 . The reduction condition of the catalyst is: H 2 / N 2 =5 / 95, temperature 300°C, pressure 0.1MPa, space velocity 2400h -1 ; Reaction conditions: H 2 / CO 2 =3, temperature 250°C, pressure 3.0MPa, W / F 10g·h / mol. ...
Embodiment 2
[0029] Graphene oxide was prepared by the modified Hummers method, first adding concentrated sulfuric acid (H 2 SO 4 , 115mL, 98%) was cooled in an ice bath. When the temperature of the system was lower than 5°C, flake graphite (5 g) was added. Stir for 30min, mix well, slowly add potassium permanganate (KMnO 4 , 15g), control the temperature of the reaction solution not to exceed 5°C, and stir for 1h. Then place the beaker in a constant temperature water bath at 30°C, continue to stir for 1 h, then add 230 mL of deionized water, and simultaneously add H 2 o 2 (30% aqueous solution, 25 mL), the solution changed from brick red to bright yellow, and the reaction was terminated. After standing for 12 hours, it was washed with dilute HCI (1:10 / volume ratio, 2L), frozen at -18°C, and then dried at -56°C using a freeze dryer to obtain a graphite oxide sample.
[0030] Measure 200mL of deionized water and dissolve it as solution A. Weigh 0.20g GO, press Cu / ZnO / ZrO 2 =4 / 3 / 3 ma...
Embodiment 3
[0032] Graphene oxide was prepared by the modified Hummers method, first adding concentrated sulfuric acid (H 2 SO 4 , 115mL, 98%) was cooled in an ice bath. When the temperature of the system was lower than 5°C, flake graphite (5 g) was added. Stir for 30min, mix well, slowly add potassium permanganate (KMnO 4 , 15g), control the temperature of the reaction solution not to exceed 5°C, and stir for 1h. Then place the beaker in a constant temperature water bath at 30°C, continue to stir for 1 h, then add 230 mL of deionized water, and simultaneously add H 2 o 2 (30% aqueous solution, 25 mL), the solution changed from brick red to bright yellow, and the reaction was terminated. After standing for 12 hours, it was washed with dilute HCl (1:10 / volume ratio, 2L), frozen at -18°C, and dried at -56°C using a freeze dryer to obtain a graphite oxide sample.
[0033] Measure 200mL of deionized water and dissolve it as solution A. Weigh 0.35g GO, press Cu / ZnO / ZrO 2 =4 / 3 / 3 mass ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com