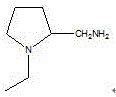
Process for producing 1-ethyl-2-aminomethylpyrrolidine
A technology of aminomethylpyrrolidine and nitromethylenepyrrolidine is applied in the field of 1-ethyl-2-aminomethyl, and can solve the problem of high reaction pressure, high reaction pressure of 1-ethyl-2-aminomethylpyrrolidine, Recycling solvent wastes energy and low reaction yield, and achieves the effect of reducing side reactions, easier recovery, and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 78g (0.5mol) of 1-ethyl-2-nitromethylenepyrrolidine and 300ml of methanol into a 1000ml autoclave, then add catalyst nickel (8g), fill it with nitrogen to a pressure of 5kg, and re-exhaust After three times, add hydrogen at a hydrogen pressure of 35 kg and a temperature of about 80°C until the hydrogen pressure does not decrease. The reaction time is about 4 hours. 49 grams of colorless liquid, yield 77%, GC content ≥ 98.5%.
Embodiment 2
[0025] Add 78g (0.5mol) of 1-ethyl-2-nitromethylenepyrrolidine and 300ml of methanol into a 1000ml autoclave, then add catalyst nickel (8g), fill it with carbon dioxide to a pressure of 5kg, and exhaust the gas After three times, press the carbon dioxide pressure to 10 kg, then add hydrogen at a hydrogen pressure of 35 kg and a temperature of 80°C until the hydrogen pressure does not decrease. The reaction time is about 4 hours. , and then rectify after recovering methanol to obtain 53 grams of colorless liquid, with a yield of 83% and a GC content of ≥99%.
[0026] The following table 1 is a comparison table of the production process of 1-ethyl-2-aminomethylpyrrolidine in Examples 1 to 2 to produce 1-ethyl-2-aminomethylpyrrolidine:
[0027] Example 1 Example 2 stamping nitrogen stamping partial pressure of carbon dioxide Raw material quantity 78g 78g Yield 49g 53g yield 77% 83% GC content ≥98.5% ≥99%
[0028] As can be se...
Embodiment 3
[0030] With reference to Example 2, the catalyst nickel was replaced with 5% palladium carbon (2 grams) as a catalyst, and the other reaction conditions were unchanged. After the treatment, 55 grams of a colorless liquid were obtained, with a yield of 86% and a GC content ≥ 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com