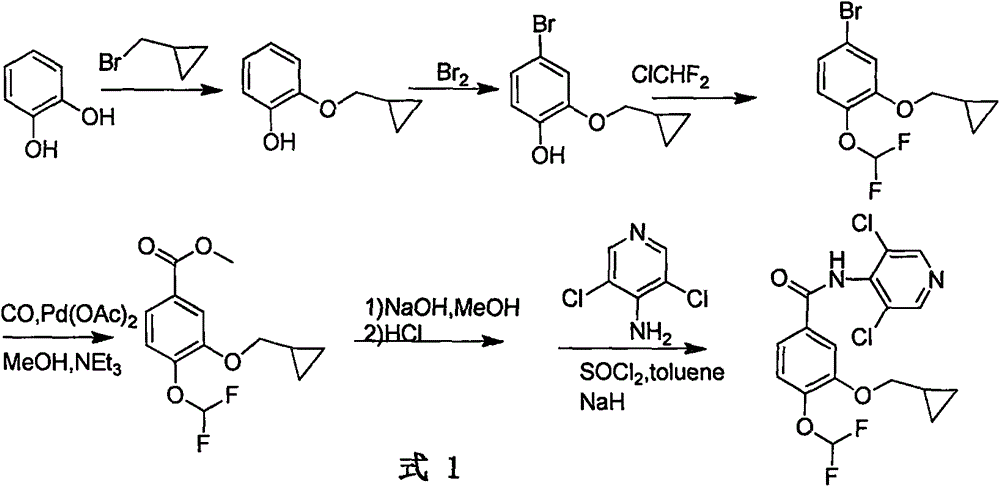
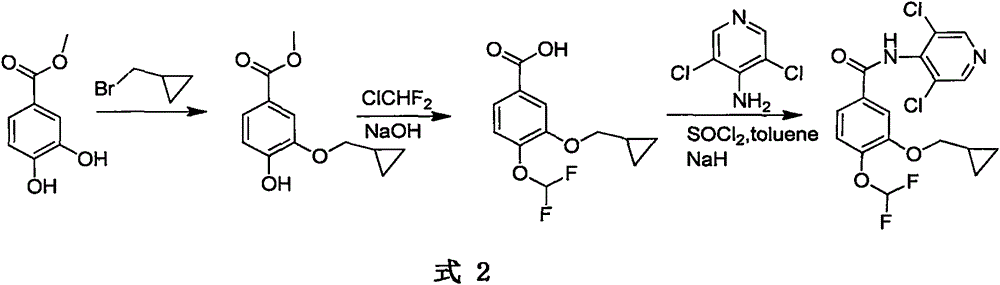
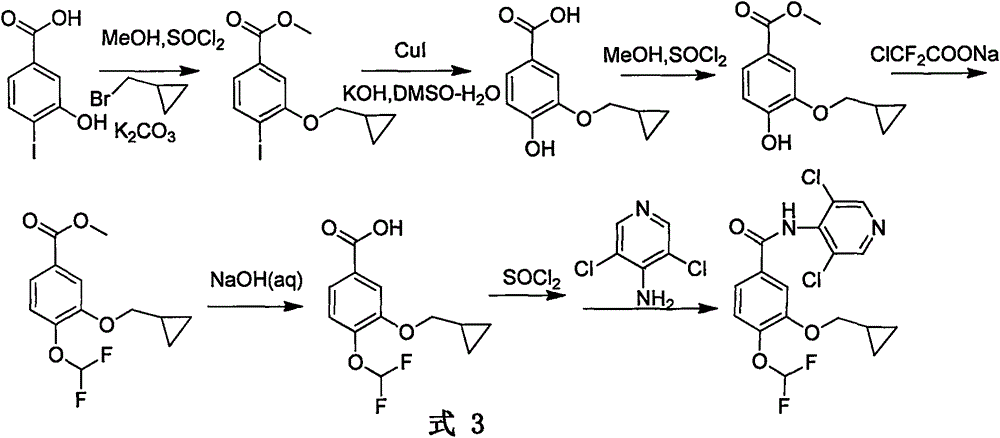
Method for preparing drug Roflumilast for treating chronic obstructive pulmonary disease
A technology for roflumilast and a drug, which is applied in the field of chemical drug synthesis, can solve problems such as difficult to obtain widely, expensive starting materials, difficult quantitative control, etc., so as to avoid the use of precious metal catalysts, shorten the synthesis process route, and reduce the The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] Example 1: Preparation of 3-hydroxyl-4-difluoromethoxybenzoic acid methyl ester
[0037] N 2 Under protection, in a 250ml three-necked bottle, 10g (59.47mmol) 3,4-dihydroxybenzoic acid methyl ester, 8.93g (89.193mmol) KHCO 3 Dissolve in 150ml DMF. Stir and slowly heat to 80°C. ClF 2 9.07g (59.47mmol) of COONa was dissolved in 60ml of DMF and added dropwise to the above reaction system. After the addition was complete, the reaction was carried out at this temperature for 6h. After cooling to room temperature, 400ml of water was added, the organic phase was extracted with 150ml of ethyl acetate x 3, and the combined organic phase was dried over anhydrous sodium sulfate. After distilling off the solvent, 7.01 g of the crude product was obtained, with a yield of 53.95%.
example 2
[0038] Example 2: Preparation of 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid methyl ester
[0039] Put 5g (22.92mmol) of methyl 3-hydroxy-4-difluoromethoxybenzoate and 8.80g (63.67mmol) of anhydrous potassium carbonate in a 250ml three-necked bottle, add 130ml of DMF to dissolve the substrate, and stir at room temperature 30min. Add 4.3g (31.85mmol) of cyclopropyl bromide in 10ml of DMF dropwise to the reaction system, and then heat to 75°C for 10h after the dropwise addition. After the reaction is completed, cool to room temperature, add 300ml of water, adjust the pH to 8-9, extract with ethyl acetate 150ml×3, combine the organic phases, and dry over anhydrous sodium sulfate. After distilling off the solvent, the crude product was recrystallized from ethyl acetate to obtain 5.82 g of a white solid, with a yield of 93.27%.
example 3
[0040] Example 3: Preparation of 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid
[0041] Dissolve 5g (18.37mmol) of methyl 3-cyclopropylmethoxy-4-difluoromethoxybenzoate in 50ml of methanol, slowly add 10ml of 30% NaOH (aq) dropwise under ice-bath conditions, dropwise After completion, react at 20-30°C for 2h. After the reaction was completed, the pH was adjusted to 4 with concentrated hydrochloric acid, extracted with 100 ml×3 ethyl acetate, the organic phase was collected, and dried by adding anhydrous sodium sulfate. After distilling off the solvent under reduced pressure, 4.6 g of the target product was obtained as a white solid, with a yield of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com