Preparation method of 3, 4-dihydropyrimidine-2-ketone derivative through high-efficient catalyzation of ethyl alcohol promoted titanocene dichloride
A technology of titanocene dichloride and dihydropyrimidine, which is applied in the field of efficient preparation of 3,4-dihydropyrimidin-2-one derivatives, and can solve complex catalyst preparation process, tedious experimental process, narrow substrate application range, etc. problems, to achieve the effect of stable air, less dosage, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
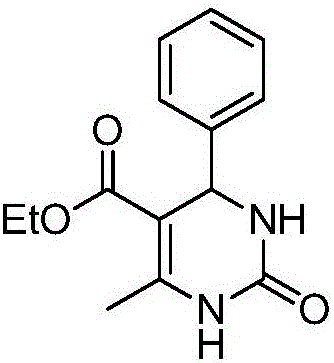
[0012] Preparation of 5-ethoxyformyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2-one of the following structural formula
[0013]
[0014] Add 0.0250 g (0.1 mmol) titanocene dichloride, 102 μL (1 mmol) benzaldehyde, 128 μL (1 mmol) ethyl acetoacetate, 0.120 g (2 mmol) urea, 4 mL ethanol to a 50 mL Shrek tube, and stir at 70 °C Reacted for 10 hours, stopped the reaction, added 15mL of dichloromethane, removed the dichloromethane by rotary evaporation, separated with a silica gel column (the eluent was a mixture of ethyl acetate and sherwood oil in a volume ratio of 1:1), and obtained 5- Ethoxyformyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2-one, its yield is 93%, and the spectral data of the product are: 1 H NMR (400MHz, DMSO) δ9.19(s,1H),7.74(s,1H),7.34–7.29(m,2H),7.24(d,J=6.9Hz,3H),5.15(d,J= 3.1Hz, 1H), 3.98(q, J=7.1Hz, 2H), 2.25(s, 3H), 1.09(t, J=7.1Hz, 3H); 13 C NMR (101 MHz, DMSO) δ 165.39, 152.26, 148.38, 144.92, 128.42, 127.30, 126.31, 99.35, 59.31, 54.06, 17.83, 14.09....
Embodiment 2
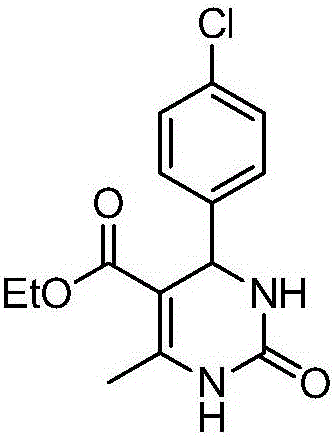
[0020] Preparation of 4-(4-chlorophenyl)-5-ethoxyformyl-6-methyl-3,4-dihydropyrimidin-2-one of the following structural formula
[0021]
[0022] In Example 1, the benzaldehyde used is replaced with equimolar 4-chlorobenzaldehyde, and other steps are the same as in Example 1 to obtain 4-(4-chlorophenyl)-5-ethoxyformyl-6-methanol Base-3,4-dihydropyrimidin-2-one, its yield is 81%, and the spectral data of product is: 1 H NMR (400MHz, DMSO) δ9.29(s, 1H), 7.81(s, 1H), 7.37(d, J=8.3Hz, 2H), 7.28(d, J=8.3Hz, 2H), 5.19(d , J=2.4Hz, 1H), 3.98(dd, J=12.4, 6.5Hz, 2H), 2.28(s, 3H), 1.08(t, J=7.0Hz, 3H); 13 C NMR (101 MHz, DMSO) δ 165.31, 152.22, 148.76, 143.88, 131.98, 128.38 (d, J=14.7 Hz), 99.04, 59.36, 53.63, 17.91, 14.11.
Embodiment 3
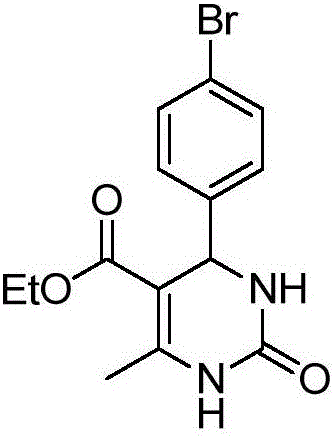
[0024] Preparation of 4-(4-bromophenyl)-5-ethoxyformyl-6-methyl-3,4-dihydropyrimidin-2-one of the following structural formula
[0025]
[0026] In Example 1, the benzaldehyde used is replaced with equimolar 4-bromobenzaldehyde, and other steps are the same as in Example 1 to obtain 4-(4-bromophenyl)-5-ethoxyformyl-6-methanol Base-3,4-dihydropyrimidin-2-one, its yield is 90%, and the spectral data of product is: 1 H NMR (400MHz, DMSO) δ9.30(s, 1H), 7.81(s, 1H), 7.51(d, J=8.0Hz, 2H), 7.23(d, J=8.2Hz, 2H), 5.17(d ,J=12.4Hz,1H),3.98(dd,J=13.2,6.4Hz,2H),2.28(s,3H),1.08(t,J=6.9Hz,3H); 13 C NMR (101 MHz, DMSO) δ 165.30, 152.24, 148.75, 144.29, 131.38, 128.68, 120.48, 99.00, 59.38, 53.71, 17.94, 14.13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com